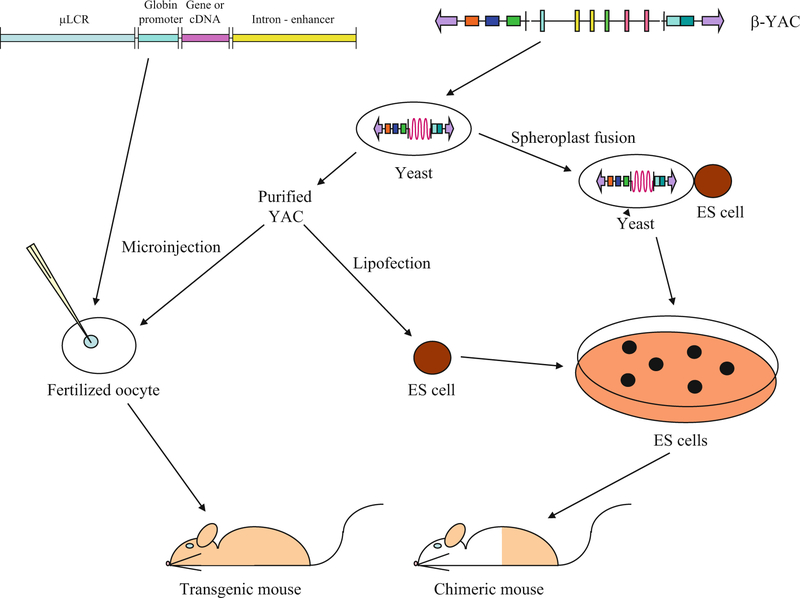
Fig. 1.
Transgenesis with small recombinant or whole loci globin constructs. A prototypical small recombinant globin transgene is illustrated at the upper left. Most early transgenes consisted of LCR sequences (mini- or micro-LCRs, one or more 5′HSs, or the entire LCR), a globin promoter linked to its gene sequences, hybrid globin gene sequences, or a cDNA reporter (occasionally a non-globin promoter was linked to a globin gene or cDNA cassette), and a globin or heterologous intron and/or enhancer (or not). These purified transgenes were microinjected into the pronucleus of fertilized mouse oocytes to produce transgenic mice as shown schematically. Cosmid and bacterial artificial chromosomes (BACs) containing larger β-like globin locus inserts were similarly microinjected (not shown). Methods used to generate transgenic mice with yeast artificial chromosomes (YACs) are illustrated beginning with the human β-globin locus YAC (β-YAC) shown at the upper right. The β-YAC (155 or 215 kb) and other YACs ranging up to 650 kb in size may be purified from the yeast host and microinjected similar to small recombination DNA constructs or lipofected into embryonic stem (ES) cells, which are then utilized to produce chimeric mice and establish transgenic lines. Alternately, the YAC may be transferred to ES cells by yeast spheroplast-ES cell protoplast fusion. Lipofection or fusion must be employed when the YAC exceeds 650–800 kb in size