Abstract
Objective
The aim of this study was to evaluate the histopathological features of primary extremity myxoid liposarcoma before and after neoadjuvant radiation therapy, and to evaluate the oncological outcomes of the patients.
Methods
The study included 23 patients (16 men and 7 women with a mean age of 43 (24–69) years) with primary myxoid liposarcoma of the extremities, who were treated between January 1998 and December 2015. Inclusion criteria were histopathological confirmation of the diagnosis with both the initial biopsy and the resection specimen, and having undergone neoadjuvant radiotherapy. Demographic, clinical and histopathological data were evaluated.
Results
Over a mean follow-up time of 55.2 (8–139) months, 5 patients (21.7%) died secondary to disease progression, leaving 18 patients (78.3%) still alive at the time of last follow-up. Only one patient (4%) experienced local recurrence and six (26%) patients developed distant metastases. Disease-free survival at 5 and 10 years were 66%; whereas, overall patient survival at 5 and 10 years were 78.1% and 71.0%, respectively. Tumor size (>15 cm) and presence of metastasis were significantly associated with increased overall mortality. On histopathology, necrosis was present in 12/23 resection specimens. Hyalinization/fibrosis and residual viable tumor was present in all specimens. Adipocytic maturation/cytodifferentiation was seen in 8/23 patients.
Conclusion
Neoadjuvant radiotherapy was effective for myxoid liposarcomas histopathologically, although these histopathological features did not affect the patients' oncological outcomes. Favorable oncological outcomes were obtained with neoadjuvant radiotherapy, surgical resection and chemotherapy.
Level of evidence
Level IV, therapeutic study.
Keywords: Neoadjuvant radiotherapy, Myxoid liposarcoma, Histopathology, Outcomes
Introduction
Liposarcoma (LPS) is the most common type of soft tissue sarcoma (STS) of in adults, accounting for 15% to 25% of all sarcomas.1 The World Health Organization (WHO) divides LPS into five distinct subtypes: atypical lipomatous tumor/well-differentiated LPS, dedifferentiated LPS, myxoid/round cell LPS, pleomorphic LPS, and LPS not otherwise specified.2 In the revised 2013 WHO classification, the term round cell LPS has been replaced with myxoid LPS, however, it is still given as a synonym.2
The LPS subtypes vary widely in their histological appearance and biological behavior—for example, while atypical lipomatous type has a good prognosis and no metastatic potential, high-grade myxoid and pleomorphic LPS subtypes have a poor prognosis and high metastatic rate.3 Myxoid liposarcoma accounts for 15–20% of all liposarcomas and represents 5% of all soft tissue sarcomas in the adults.2 In this study, we aimed to study the effectiveness of neoadjuvant therapy and oncological outcomes in a group of myxoid liposarcoma patients.
The preferred treatment for extremity STS is limb-sparing surgery. However, adjunct radiation therapy has an increasingly important role in the treatment of STS.3 Although RT for extremity STS can be performed in both the pre- and post-operative settings, potential advantages of pre-operative RT include decreased rates of late complications, lower radiation doses, and the potential to improve resectability prior to surgery.3 Because of this, the use of neoadjuvant radiation therapy with or without chemotherapy has become common in STS, including for most subtypes of LPS (with the exception of atypical lipomatous tumor, which can generally be managed with surgery alone). In particular, myxoid LPS are relatively radiosensitive when compared to other STS subtypes.3
The assessment of radiologic response to treatment with RT in LPS can be challenging. While traditional response criteria for solid tumors have relied on decreases in tumor size,3 some studies suggest that pathologic response to RT in sarcomas may occur without a change in size, or even with a size increase in certain cases.4, 5, 6 In LPS specifically, there are very few studies examining the imaging appearance and histopathology following RT.7, 8 Accordingly, the purpose of our study was to evaluate the histopathological features of primary extremity myxoid LPS before and after neoadjuvant radiation therapy, and compare oncological outcomes of the patients.
Material and methods
This study was approved by the Institutional Review Board. We identified 124 patients with primary extremity LPS treated in our university clinic between January 1998 and December 2015. The electronic medical records of all 124 patients were reviewed looking for the following inclusion criteria: (i) Primary myxoid liposarcoma of the extremities as a histological diagnosis, (ii) treatment with neoadjuvant radiation therapy with or without chemotherapy, (iii) histological investigation of both biopsy and resection specimens obtained in our institution prior to and after neoadjuvant radiotherapy respectively and (iv) at least one baseline MRI. Of the 124 patients, 23 patients fulfilled the criteria and were included in the study. Demographic and clinical data for each patient was extracted, including gender, age, date of diagnosis, dates of radiation therapy, dose of radiation therapy, date of surgical resection, and presence of recurrence and metastasis (at presentation or follow-up) (Table 1). Our radiotherapy protocol consisted of hypo-fractioned radiotherapy (28Gy/8fr). Pre-operative chemotherapy was administered to 8 patients (34.8%) and there was no patient with post-operative chemotherapy in this series. The standard chemotherapy regimen was 2 cycles of Adriamycin and Ifosfamide.
Table 1.
Characteristic of 23 Patients with primary myxoid liposarcoma in the extremities.
| Characteristic | N (%) = 23 |
|---|---|
| Age, years, mean (Range) | 43 (24–69) |
| Gender, male (%) | 16 (69.6) |
| Tumor location, N (%) | |
| Upper extremities | 1 (4.3) |
| Lower extremities | 22 (95.6) |
| Tumor Size, N (%) | |
| Mean (cm) (Range) | 13 (5–30) |
| <15 cm | 14 (60.9) |
| ≥15 cm | 9 (39.1) |
| Original Margins, N (%) | |
| Wide | 19 (82.6) |
| Marginala | 4 (17.4) |
| Neoadjuvant Chemotherapy, N (%) | 8 (34.7) |
| Neoadjuvant Radiotherapyb | |
| Dose | 28 Gy |
| Number of fractions | 8 |
Marginal resection was limited to preservation of neurovascular structures in 4 for patients. Rest of the specimen had wide resection margins.
All patients were treated with radiotherapy had the same dose and fractions.
Histopathological examination
Pathology reports of both biopsy and resection specimens for each primary tumor were reviewed from the medical records. Except 2 patients, who have undergone incisional biopsy, all patients have undergone biopsy with a Tru-cut needle. Pathology specimens were reexamined by the two pathologist experienced musculoskeletal pathology. Following variables were extracted: tumor size, tumor grade, margin, presence of round cells, presence and percentage of necrosis, presence and percentage of hyalinization/fibrosis, and the percentage of remaining viable tumor and vascularization patterns. The treatment response was defined as the sum of the percentages of hyalinization/fibrosis and necrosis.
Excision specimens were examined on paraffin embedded blocks. The number of paraffin blocks for each specimen was related to the size of the tumor, with approximately one additional block for each cm of specimen diameter. Hyalinization/fibrosis and necrosis percentages were estimated for each paraffin block and agreed upon by the pathologists. The final percentage values for hyalinization/fibrosis and necrosis were calculated from the average of all paraffin blocks of the specimen and were added together to get a semi-quantitative value for treatment response percentage. Surgical margins were classified as wide, marginal or intra-lesional according to pathology reports.
Kaplan–Meier and Cox proportional hazards regression analyses were used to examine the risk of local recurrence,9, 10, 11, 12 distant metastasis,13 disease-free survival (DFS), and overall survival (OS) according to patient age, gender, tumor and treatment characteristics. Histopathological features of the biopsy and excision specimens were evaluated by the Chi-square test and Wilcoxon tests.
Results
The study cohort consisted of sixteen men and seven women with a mean age of 43 years (range 24–69 years). All tumors underwent surgical resection, which occurred at a median of 16 days following completion of neoadjuvant therapy (range 6–30 days). Average tumor size upon resection was 13.7 cm (range 5–30 cm).
Histopathological outcomes
Surgical margins were wide in 19 (82.6%) patients; however, in 4 patients (17.4%) in order to preserve important neurovascular structures, marginal resection was performed around these structures.
Although initial diagnostic biopsy samples revealed round cell components in 8 (34.8%) cases, round cells were observed in the excision specimens of only 5 cases (21.7%). The decrease in cases with round cell component was found to be significant (p: 0.016) (Fig. 1). The mean round cell percentage in the excision specimens was 11% for those 5 cases. The excision specimens of the remaining 18 cases were observed to have pure myxoid histology. Although resection specimens revealed round cells in 3 out of 6 cases with metastatic tumors, there was no statistically significant correlation between the presence of round cells and development of metastasis (p: 0.054).
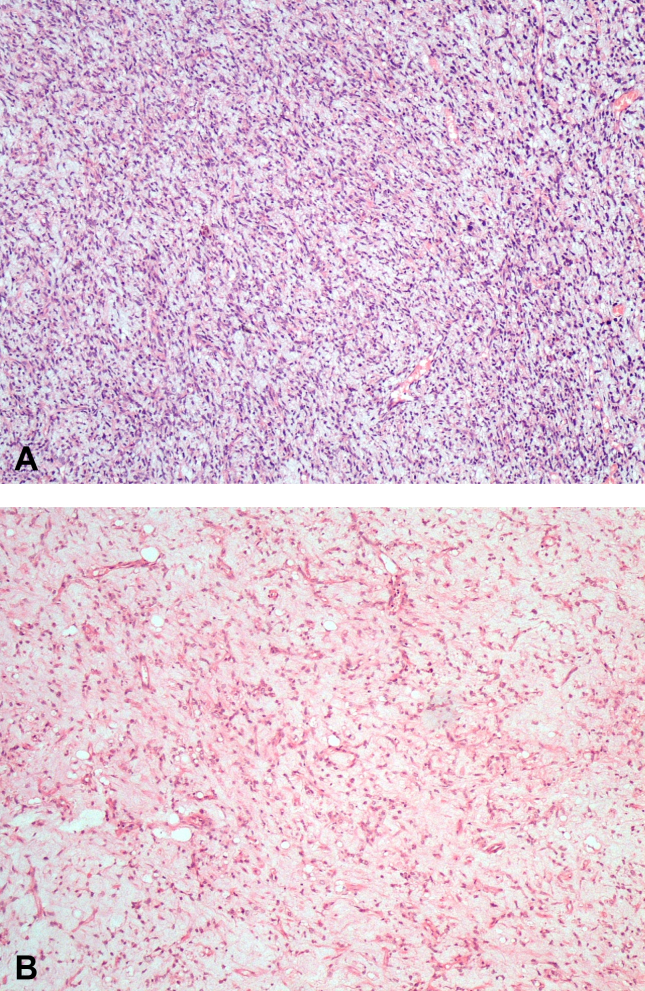
Fig. 1.
1A: Biopsy image of a 67-year-old male (patient no. 20) with myxoid liposarcoma shows rich round cell component. 1B: Microscopic appearance of post-radiotherapy excision specimen of the same patient. Round cell component is decreased (HEx100).
On histopathology, necrosis was present in 12 of 23 (52.2%) resection specimens (Fig. 2). Hyalinization/fibrosis was present in all resection specimens with 15 out of 23 (65.2%) cases having 50% or greater hyalinization/fibrosis (Fig. 3). Mean treatment response was 67.8% (range: 30–90%). Treatment response was equal to or greater than 90% in 6 out of 23 (26%) patients. However, it was not correlated with oncological outcomes.
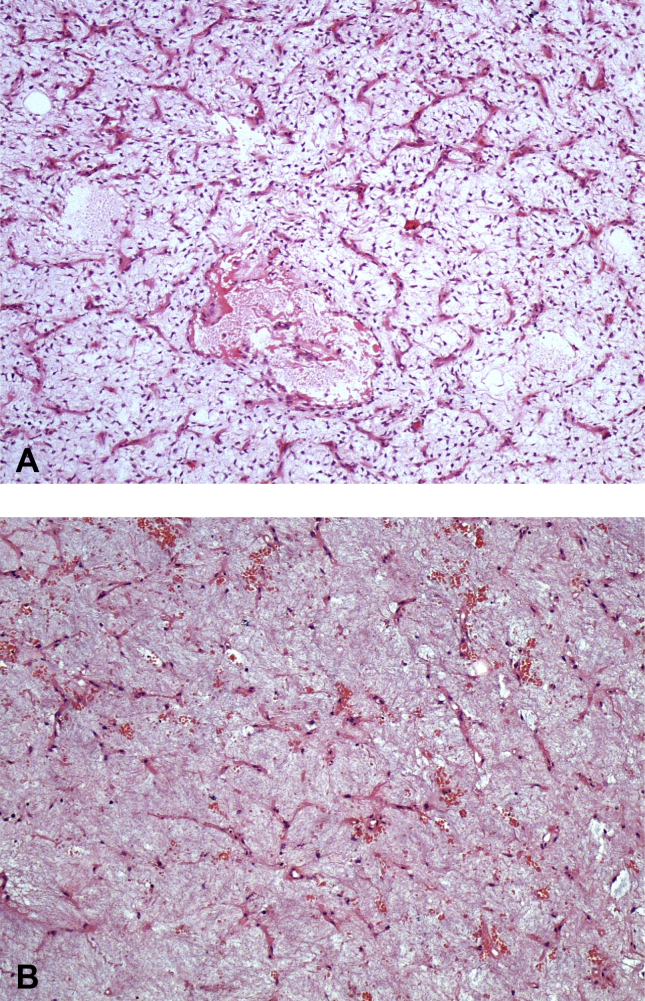
Fig. 2.
2A: Biopsy image of a 34-year-old male (patient no. 16) with myxoid liposarcoma at the lower extremity before radiotherapy. Plenty of plexiform vascular structures are apparent. In between the vascular structures are spindle/stellate tumor cells. Round cell component is absent. (HEx100) 2B: Microscopic appearance of the excision from the same case after radiotherapy. Vascular structures are diminished. The tumoral cells are mostly decreased as well (HEx100).
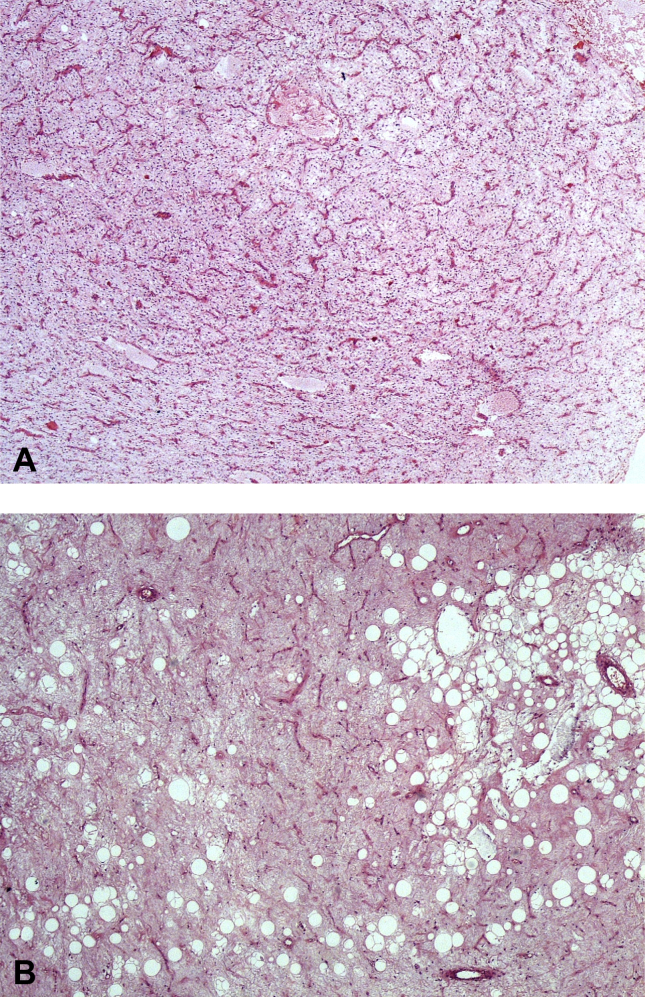
Fig. 3.
3A: Biopsy image of a 51-year-old male (patient no. 21) with myxoid liposarcoma located at the lower extremity. Typical microscopic appearance of myxoid liposarcoma with a myxoid background and rich vascular structures with tumor cells in between. (HEx40). 3B: Microscopic appearance of the excision from the same case. Tumor cells and vascular structures are diminished. Adipocyte maturation is a striking feature. (HEx100).
Residual viable tumor was present in all resection specimens; while residual viable tumor component was less than 50% in 18 out of 23 (78.3%), this ratio was 50% or greater in remaining 5 (21.7%) cases. Extensive (90%) hyalinization with only 0–10% residual viable tumor was observed on histopathology in 3 patients.
Histopathology revealed adipocytic maturation/cytodifferentiation in 8 out of 23 patients. The histopathological features of all resection specimens along with age and sex information of the patients are displayed in Table 2.
Table 2.
Summary of histopathological and oncological features of patients.
| Order | Oncologic Status | Follow-up (mo) | Age | Sex | Round cell component % | Necrosis % | Hyalinization/Fibrosis % | Viable tumor % | Fat maturation % | Round cell component in the biopsy % |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NED | 139 | 39 | M | - | 30 | 60 | 10 | + | 10 |
| 2 | NED | 134 | 32 | M | – | 10 | 60 | 30 | 10 | |
| 3 | NED | 9 | 35 | F | – | 0 | 80 | 20 | – | |
| 4 | NED | 86 | 27 | F | – | 20 | 40 | 40 | – | |
| 5 | NED | 79 | 29 | F | – | 10 | 60 | 30 | – | |
| 6 | NED | 43 | 53 | F | – | 0 | 30 | 70 | + | – |
| 7 | DOD | 11 | 30 | M | 10 | 0 | 30 | 70 | 10 | |
| 8 | DODa | 64 | 56 | F | – | 10 | 80 | 10 | – | |
| 9 | NED | 11 | 24 | M | – | 0 | 80 | 20 | + | – |
| 10 | DOD | 13 | 53 | M | – | 20 | 60 | 20 | + | – |
| 11 | NED | 91 | 27 | M | 5 | 0 | 90 | 10 | 10 | |
| 12 | NED | 83 | 58 | M | – | 10 | 50 | 40 | + | 50 |
| 13 | DOD | 40 | 34 | M | – | 30 | 30 | 40 | – | |
| 14 | NED | 79 | 48 | F | – | 0 | 90 | 10 | – | |
| 15 | NED | 79 | 55 | M | – | 10 | 30 | 60 | – | |
| 16 | DOD | 31 | 69 | M | 10 | 40 | 30 | 30 | 10 | |
| 17 | NED | 15 | 67 | M | 40 | 20 | 10 | 70 | 40 | |
| 18 | NED | 31 | 51 | M | – | 0 | 80 | 20 | + | – |
| 19 | NED | 99 | 45 | M | – | 0 | 30 | 70 | + | 10 |
| 20 | NED | 32 | 47 | F | – | 0 | 70 | 30 | – | |
| 21 | AWD | 71 | 49 | M | 5 | 0 | 90 | 10 | – | |
| 22 | NED | 21 | 33 | M | – | 10 | 80 | 10 | + | – |
| 23 | NED | 8 | 30 | M | – | 0 | 80 | 20 | – |
NED: no evidence of disease; AWD: Alive with disease; DOD: dead of disease.
This patient developed local recurrence. It was treated with re-excision, however the patient died due to metastases at 64 months after the initial treatment.
Survival and oncological outcomes
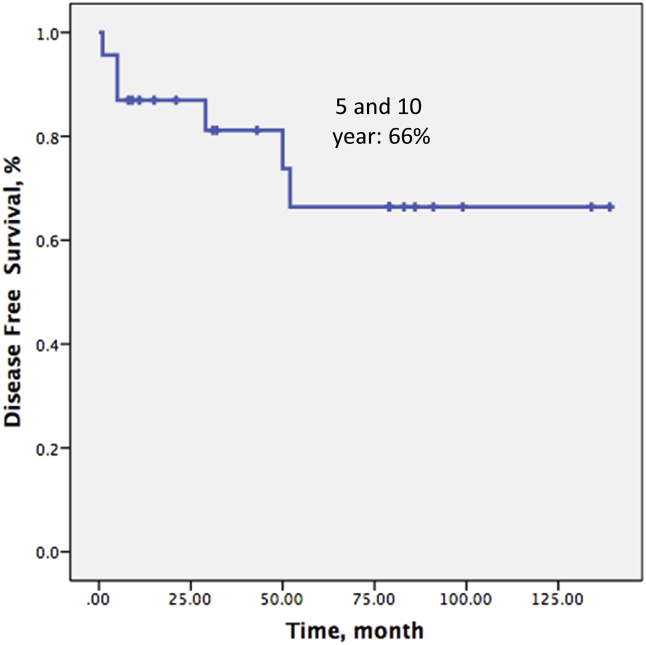
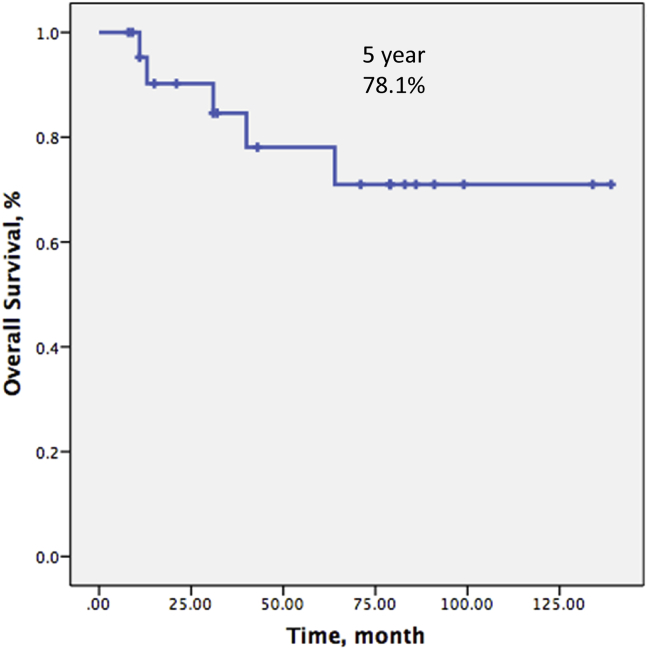
Over a mean follow-up time of 55.1 (8–139) months, 5 patients (21.7%) died secondary to disease progression, leaving 18 patients (78.3%) still alive at the time of last follow-up. Only one patient (4%) experienced local recurrence. Six (26%) patients developed distant metastases. Disease-free survival at 5 and 10 years were both 66% (Fig. 4) whereas overall patient survival at 5 and 10 years were 78.1% and 71.0%, respectively (Fig. 5).
Fig. 4.
Kaplan–Meier curves show disease-free survival of the patients.
Fig. 5.
Kaplan–Meier curves show 5-year overall survival of the patients.
The time to local recurrence from surgical treatment was 52 months in the single case with LR. The recurrent tumor was treated with re-excision, however the patient died due to metastases at 64 months after the initial treatment. Margin status of this patient was wide in the initial resection. Recurrence-free survival was found to be 91% at both 5 and 10 years for this patient series.
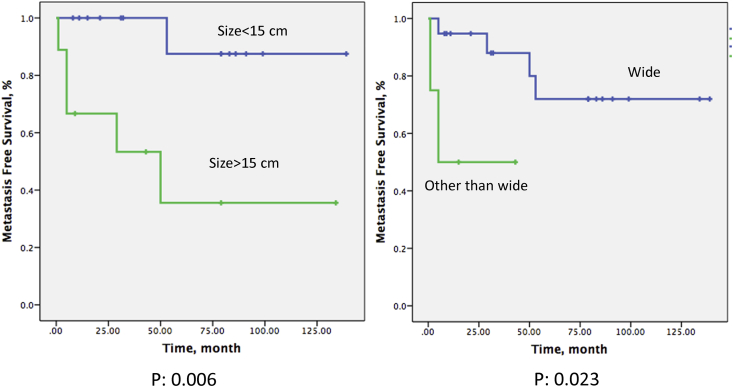
The mean time to metastasis was 51 (1–139) months for the 6 patients, who experienced distant metastases. Initial site of distant disease was predominantly the lung. The mean tumor size at presentation was 13.6 (5–30) centimeters for those with distant metastases, (One patient with distant metastases was still alive 50 months after the detection of metastatic disease while the remaining 5 patients died at a mean of 13.5 (2–35) months. Of the 6 patients with metastases, five had a tumor size greater than 15 cm and tumor size was found to correlate significantly with the development of metastases in these cases (p: 0.006). Wide surgical margin was also associated with higher metastases free survival comparing other than wide margin (p: 0.023) (Fig. 6). Other variables did not yield any significant correlation with metastasis. Round cells were present in resection specimens in 3 out of 6 metastatic cases. While 5-year metastasis free survival of the patients with round cells was 27%, it was 79% for patients without round cells in the resection specimens (p: 0.052). Metastasis-free survival at 5 years and 10 years were both 66%.
Fig. 6.
Effect of tumor size and margin status on metastasis-free survival.
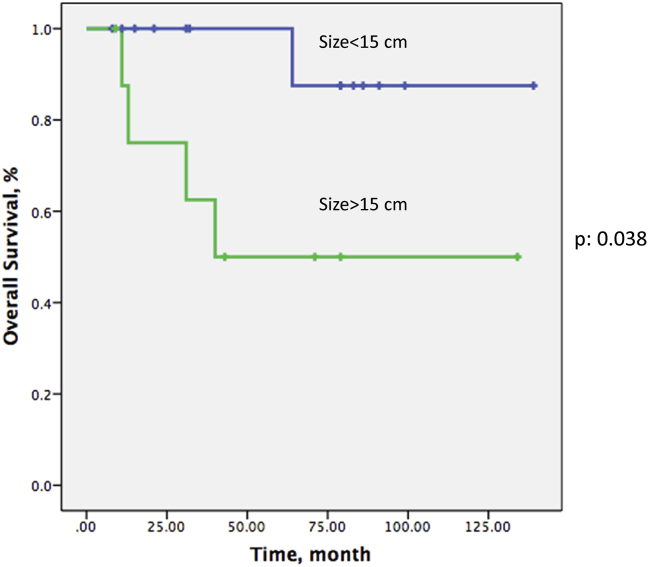
Tumor size greater than 15 cm was associated with significantly increased overall mortality (p: 0.038) (Fig. 7). Metastasis was significantly associated with overall survival (p: 0.001). Although 5-year overall survival was 68% in the poor response group, it was 80% in the good response group. However, this association was not statistically significant. Additionally, round cell component of the tumor was not found to correlate significantly with overall survival in this patient series. Overall survival at 5 years was 85% for pure myxoid LPS cases while it was 53% for round cell/myxoid LS cases, however, this difference was not statistically significant (p > 0.05). Age, sex, marginal status of resection specimen, presence and proportion of round cell component and other histological parameters were not found to correlate with overall survival.
Fig. 7.
Effect of tumor size on overall survival.
When we compared patients with neoadjuvant radiotherapy and patient with neoadjuvant radiotherapy and chemotherapy, there was no statistical difference between two groups in terms of histological and oncologic parameters.
Discussion
Myxoid cell liposarcomas occur mostly in middle-aged adults primarily as extremity lesions. Histopathological features of tumors range from pure myxoid (low grade) to pure round cell (high-grade lesions) with some cases having transitional features. Tumor behavior may be related to the proportion of round cell areas.9, 14, 15, 16
Neoadjuvant radiotherapy is commonly utilized in patients with myxoid LPS, and several studies have shown that myxoid LPS are extremely radiosensitive.17, 18, 19, 20 The effect of radiotherapy can be explained by several mechanisms: decrease in myxoid stroma produced by tumor cells, vascular damage and adipocyte maturation. There are two different hypotheses concerning adipocyte maturation after radiotherapy: relative predominance of radioresistant cells such as adipocytes, which show a low turn-over rate, over radiosensitive cells such as tumor cells, which show a high turn-over rate, after radiotherapy and secondly, radiation induced tumor differentiation. We have also observed these histopathological changes after radiotherapy. Another effect of radiotherapy is the change in tumor size. Consistent decreases in tumor volume have been reported with myxoid LPS21; however, for pleomorphic sarcomas, some studies even report increases in volume.9, 14, 15, 16 We could not perform a post-radiotherapy volumetric evaluation with MRI in all patients. A cut-off value of 90% for treatment response was set for comparing oncological outcomes. Although, to our knowledge, no such accepted threshold value exists for LPS, one exists for osteosarcoma.12, 16 However, the 90% cut-off value did not yield statistically significant results in terms of oncological outcomes. This may have resulted from our relatively small sample size. The number of cases with a round cell component was lower in the excision specimens. We believe this may be the result of neoadjuvant treatment.
Our radiotherapy treatment protocol for myxoid liposarcoma consisted of fractionated (28Gy/8fr) radiotherapy, which results in a different treatment response than the conventional radiotherapy in terms of especially acting on the intima of the vascular structures. The main advantages of the hypo-fractionated radiotherapy is that surgery can follow without delay and local wound complications are decreased compared to conventional radiotherapy.14
In our study, local control was excellent with 91% of cases having no local recurrence at 5-years. Rate of local recurrence in this study (4%) was favorable compared to rates of local recurrence in the literature, which vary between 3 and 33%.14, 15, 16, 22 Although Eilbert et al16 demonstrated that treatment induced necrosis in high-grade soft tissue sarcomas correlated with low local recurrence rate and high survival, we did not find such a correlation in this study. Our distant control rate (26%) was similar to other studies in the literature.2 Tumor size (>15 cm) was significantly associated with decreased metastasis-free survival and overall survival. This is also in accordance with other studies in the literature.5, 8 Overall survival rates (78%) were similar with the rates in the literature, which range from 70 to 92%.11, 20, 21, 23, 24, 25, 26 However, histological parameters such as necrosis and round cell percentage of the tumor did not correlate with survival rates.
Although most patients in this series underwent histologically confirmed wide resection and had good histological response to radiotherapy, 6 out of 23 patients developed metastasis. Factors affecting the development of metastasis are still being debated. Margin status, tumor size and histological grade are commonly cited as factors associated with increased risk of distant metastasis in the literature.27 We have also shown that tumor size and marginal status were significantly correlated with metastases free survival.
The retrospective nature of evaluation and a relatively small patient cohort could be mentioned as limitations of this study. Additionally, conducting the study in a clinic, which is a tertiary care center, might have caused a bias due to referred patients having larger or more aggressive tumors. One of the inclusion criteria for this study was the presence of both biopsy and excision specimens. One of our key variables was the presence of round cells. Although inadequate representation of tumor histology due to small size of needle biopsy specimen may be stated as a weak point of this study, this is actually a common issue of debate for all musculoskeletal tumors with heterogeneous histology. Use of neoadjuvant chemotherapy in only some patients (8/23) while all had neoadjuvant radiotherapy is a potential shortcoming of the study in terms of histological evaluation of treatment response in resection specimens. Since preoperative radiotherapy is the standard protocol for myxoid liposarcomas in our institution, our study lacks a control group consisting of patients with preoperative chemotherapy only. Therefore it is difficult to make a direct comparison between the histological effects of radiotherapy and chemotherapy. On the other hand, similar histological and oncological outcomes, for patient groups with radiotherapy alone and radiotherapy and chemotherapy combined, may suggest that the main effect belongs to radiotherapy. Nevertheless, a uniform radiotherapy protocol for all patients can be regarded as a strong aspect of this study.
Another limitation of the study is the lack of a control group without any neoadjuvant treatment against which the necrosis rate could be compared. Although same studies in the literature report outcomes of patients who only received adjuvant treatment, these studies also lack necrosis rates which could be used as reference.28, 29
In conclusion, based on our data, we suggest the use of neoadjuvant therapy in patients with myxoid LPS. In conformity with the current practice, the use of neoadjuvant radiotherapy is strongly recommended. Furthermore, significant consideration should be given to neoadjuvant chemotherapy. Significant changes in tumor histopathology were observed with neoadjuvant therapy owing to different mechanisms. Favorable oncological outcomes were obtained with our treatment protocols. Local tumor control was especially successful with only one of the patients having a local recurrence.
On the other hand, further studies are needed to demonstrate the relationship between histopathological features and oncological outcomes.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Goldblum J.R., Weiss S.W., Folpe A.L. Elsevier Health Sciences; 2013. Enzinger and Weiss's soft tissue tumors. [Google Scholar]
- 2.Fletcher C.D.M., Unni K.K., Mertens F., editors. World Health Organization Classification of Tumours. IARC Press; Lyon: 2002. (Pathology and Genetics of Tumours of Soft Tissue and Bone). [Google Scholar]
- 3.Peterson J.J., Kransdorf M.J., Bancroft L.W., O'Connor M.I. Malignant fatty tumors: classification, clinical course, imaging appearance and treatment. Skeletal Radiol. 2003;32(9):493–503. doi: 10.1007/s00256-003-0647-8. [DOI] [PubMed] [Google Scholar]
- 4.DeLaney T.F. Radiation therapy: neoadjuvant, adjuvant, or not at all. Surgical Oncol Clin N Am. 2012;21(2):215–241. doi: 10.1016/j.soc.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Haas R.L.M., DeLaney T.F., O'Sullivan B. Radiotherapy for management of extremity soft tissue sarcomas: why, when, and where? Int J of Radiat Oncol Biol Phys. 2012;84(3):572–580. doi: 10.1016/j.ijrobp.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 6.DeLaney T.F., Spiro I.J., Suit H.D. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J of Radiat Oncol Biol Phys. 2003;56(4):1117–1127. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 7.Olsullivan B., Davis A.M., Turcotte R. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet. 2002;359(9325):2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 8.Davis A.M., O'Sullivan B., Turcotte R. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiotherapy Oncol. 2005;75(1):48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Pitson G., Robinson P., Wilke D. Radiation response: an additional unique signature of myxoid liposarcoma. Int J of Radiat Oncol Biol Phys. 2004;60(2):522–526. doi: 10.1016/j.ijrobp.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Chung P.W.M., Deheshi B.M., Ferguson P.C. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma. Cancer. 2009;115(14):3254–3261. doi: 10.1002/cncr.24375. [DOI] [PubMed] [Google Scholar]
- 11.Guadagnolo B.A., Zagars G.K., Ballo M.T. Excellent local control rates and distinctive patterns of failure in myxoid liposarcoma treated with conservation surgery and radiotherapy. Int J of Radiat Oncol Biol Phys. 2008;70(3):760–765. doi: 10.1016/j.ijrobp.2007.07.2337. [DOI] [PubMed] [Google Scholar]
- 12.de Vreeze R.S.A., de Jong D., Haas R.L., Stewart F., van Coevorden F. Effectiveness of radiotherapy in myxoid sarcomas is associated with a dense vascular pattern. Int J of Radiat Oncol Biol Phys. 2008;72(5):1480–1487. doi: 10.1016/j.ijrobp.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P., Arbuck S.G., Eisenhauer E.A. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Engström K., Bergh P., Cederlund C.-G. Irradiation of myxoid/round cell liposarcoma induces volume reduction and lipoma-like morphology. Acta Oncol. 2007;46(6):838–845. doi: 10.1080/02841860601080415. [DOI] [PubMed] [Google Scholar]
- 15.Roberge D., Skamene T., Nahal A., Turcotte R.E., Powell T., Freeman C. Radiological and pathological response following pre-operative radiotherapy for soft-tissue sarcoma. Radiotherapy Oncol. 2010;97(3):404–407. doi: 10.1016/j.radonc.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Wortman J.R., Tirumani S.H., Tirumani H. Neoadjuvant radiation in primary extremity liposarcoma: correlation of MRI features with histopathology. Eur Radiol. 2016;26(5):1226–1234. doi: 10.1007/s00330-015-3953-3. [DOI] [PubMed] [Google Scholar]
- 17.Stacchiotti S., Collini P., Messina A. High-grade soft-tissue sarcomas: tumor response assessment—pilot study to assess the correlation between radiologic and pathologic response by using RECIST and choi criteria 1. Radiology. 2009;251(2):447–456. doi: 10.1148/radiol.2512081403. [DOI] [PubMed] [Google Scholar]
- 18.Stacchiotti S., Verderio P., Messina A. Tumor response assessment by modified Choi criteria in localized high-risk soft tissue sarcoma treated with chemotherapy. Cancer. 2012;118(23):5857–5866. doi: 10.1002/cncr.27624. [DOI] [PubMed] [Google Scholar]
- 19.Miki Y., Ngan S., Clark J.C.M., Akiyama T., Choong P.F.M. The significance of size change of soft tissue sarcoma during preoperative radiotherapy. Eur J of Surg Oncol. 2010;36(7):678–683. doi: 10.1016/j.ejso.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Baxter K.J., Govsyeyev N., Namm J.P., Gonzalez R.J., Roggin K.K., Cardona K. Is multimodality therapy necessary for the management of pure myxoid liposarcomas? A multi-institutional series of pure myxoid liposarcomas of the extremities and torso. J Surg Oncol. 2015;111(2):146–151. doi: 10.1002/jso.23786. [DOI] [PubMed] [Google Scholar]
- 21.Suzan E., Hoekstra H.J., van Ginkel R.J., Bastiaannet E., Suurmeijer A.J.H. Clinicopathologic prognostic factors in myxoid liposarcoma: a retrospective study of 49 patients with long-term follow-up. Ann of Surg Oncol. 2007;14(1):222–229. doi: 10.1245/s10434-006-9043-7. [DOI] [PubMed] [Google Scholar]
- 22.Wardelmann E., Haas R., Bovée J. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European Organization for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group (EORTC–STBSG) recommendations for pathological examination and reporting. Eur J Cancer. 2016;53:84–95. doi: 10.1016/j.ejca.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Antonescu C.R., Tschernyavsky S.J., Decuseara R. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001;7(12):3977–3987. [PubMed] [Google Scholar]
- 24.Kilpatrick S.E., Doyon J., Choong P.F.M., Sim F.H., Nascimento A.G. The clinicopathologic spectrum of myxoid and round cell liposarcoma. Cancer. 1996;77(8):1450–1458. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1450::AID-CNCR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Nishida Y., Tsukushi S., Nakashima H., Ishiguro N. Clinicopathologic prognostic factors of pure myxoid liposarcoma of the extremities and trunk wall. Clin Orthop Relat Res. 2010;468(11):3041–3046. doi: 10.1007/s11999-010-1396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith T.A., Easley K.A., Goldblum J.R. Myxoid/round cell liposarcoma of the extremities: a clinicopathologic study of 29 cases with particular attention to extent of round cell liposarcoma. Am J Surg Pathol. 1996;20(2):171–180. doi: 10.1097/00000478-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Eilber F.C., Rosen G., Eckardt J. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19(13):3203–3209. doi: 10.1200/JCO.2001.19.13.3203. [DOI] [PubMed] [Google Scholar]
- 28.Oh Y.J., Yi S.Y., Kim K.H. Prognostic model to predict survival outcome for curatively resected liposarcoma: a multi-institutional experience. J Cancer. 2016;7(9):1174–1180. doi: 10.7150/jca.15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H.S., Lee J., Yi S.Y. Liposarcoma: exploration of clinical prognostic factors for risk based stratification of therapy. BMC Cancer. 2009;9(1):1. doi: 10.1186/1471-2407-9-205. [DOI] [PMC free article] [PubMed] [Google Scholar]