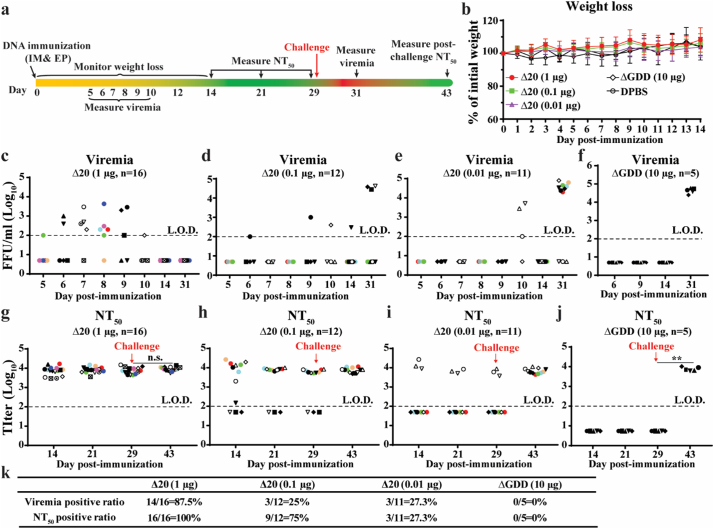
Fig. 2.
Immunization of pZIKV-3′UTR-∆20 protects the A129 mouse from ZIKV challenge. (a) Experimental design. Various doses of pZIKV-3′UTR-Δ20 (1, 0.1, 0.01 μg), pZIKV-ΔGDD (10 μg), or DPBS (sham) were inoculated to six-week-old A129 mice via intramuscular (IM) injection and electroporation (EP) using TriGrid™. Following immunization, mice were monitored for weight loss over 14 days. Since our IACUC protocol only allows four blood draws per mouse over 28 days post-transfection (or infection), blood draws were staggered for different mouse sub-cohorts to cover the sampling period of days 5–10 post-immunization. Mice were bled at indicated time for measuring neutralizing antibody titers (NT50) using an mCherry-ZIKV neutralization assay. On day 29 post-immunization, the mice were challenged with 106 PFU of ZIKV strain PRVABC59 via the subcutaneous route (indicated by a red arrow). At indicated time, the mice were bled for measuring viremia using a focus-forming assay. (b) Mouse weight post-immunization. (c-f) Viremia for the mouse groups immunized with 1 μg pZIKV-3′UTR-Δ20 (c), 0.1 μg pZIKV-3′UTR-Δ20 (d), 0.01 μg pZIKV-3′UTR-Δ20 (e), or 10 μg pZIKV-ΔGDD (f). (g-j) Neutralizing antibody titers (NT50) from the mouse groups immunized with 1 μg pZIKV-3′UTR-Δ20 (g), 0.1 μg pZIKV-3′UTR-Δ20 (h), 0.01 μg pZIKV-3′UTR-Δ20 (i), or 10 μg pZIKV-ΔGDD (j). Group sizes (n number) are indicated. Individual mice are indicated by different colors and symbols. Paired t-test was performed to indicate no significant difference (n.s.) between the pre-challenge (day 29) and post-challenge (day 43) neutralizing antibody titers in (g). (k) Summary of viremia-positive and neutralizing antibody-positive ratios for all mouse groups. Limits of detections (L.O.D., dotted lines) of focus-forming assay and neutralization assay (NT50) were 100 FFU/ml and 100-fold dilution, respectively.