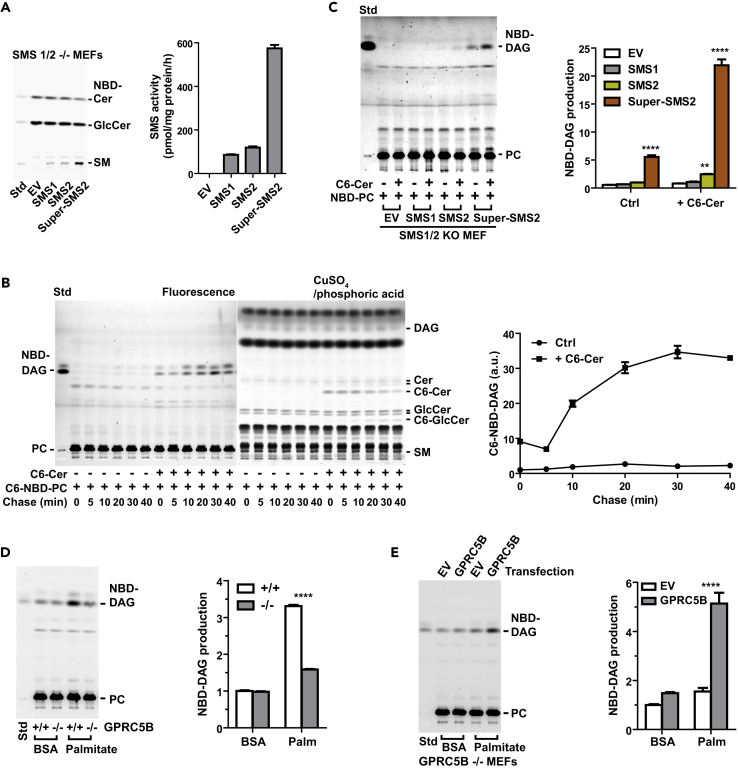
Figure 6.
Metabolic Stress Enhances SMS2-Generated DAG
(A) In vitro SMS assay. SMS2 was derived from cell lysates from SMS1/2 double-knockout MEFs transfected with the indicated constructs. Equal protein amounts of cell lysate were mixed with liposomes prepared from egg PC and NBD-Cer and then incubated in 37°C for 1 hr. Lipids were extracted from the reaction mixture and then synthesized SM was measured by high-performance thin-layer chromatography (HPTLC).
(B) Time course analysis of PC-derived DAG formation induced by the addition of exogenous C6-Cer. Cells were labeled with NBD-C6-PC alone or NBD-C6-PC plus C6-Cer for 30 min on ice, washed, and chased at the indicated times. Extracted lipids were developed via HPTLC, and fluorescence images were captured with a LAS3000 imaging system. To visualize total lipids, the same HPTLC plate was treated with cupric acid. PC-derived DAG was quantified from the fluorescence image.
(C) SMS2 expression is required for Cer-Dependent DAG formation from PC. SMS1/2 double-knockout MEFs were transfected with the indicated expression plasmids. After 48 hr of transfection, cells were labeled with NBD-PC or NBD-PC plus 5 μM C6-Cer for 30 min on ice and then washed twice and incubated at 37°C for 10 min. Fluorescence intensity of the extracted lipids was measured using a microplate reader, and the amount of lipids was determined. Same amount of fluorescent lipids was spotted on an HPTLC plate and developed.
(D) GPRC5B deficiency reduces PC-derived DAG formation under metabolic stress. Cells were cultured in medium containing BSA or palmitate-BSA complex for 16 h and then labeled with NBD-PC for 30 min on ice. After NBD-PC labeling, cells were washed twice with fresh medium and incubated at 37°C for 15 min. The same amount of fluorescent lipids was spotted onto an HPTLC plate and developed.
(E) GPRC5B enhances PC-derived DAG production by palmitate exposure. GPRC5B knockout MEFs were transfected with expression plasmid for human GPRC5B. After exposure to palmitate for 16 hr, cells were labeled with NBD-PC for 30 min on ice and then washed twice and incubated in 37°C for 15 min. NBD-DAG formation from NBD-PC were measured.
(A–E) Data are means ± SEM (n = 3; ANOVA, **p < 0.01, ****p < 0.0001).