Table 1.
Inhibitory potencies, solubility, permeability and structures of triazolo[4,5-d]pyrimidine derivatives.
| Compound |
GCN2 IC50 | PKR IC50 | % Enzyme inhibition at 10 μM |
Solubility at pH 7.4 | PAMPAa | ||||
|---|---|---|---|---|---|---|---|---|---|
| ID | Structure | (nM) | (nM) | PERK | PKR | HRI | IRE1 | (μM) | 10–6 cm/s |
| GSK2656157 | 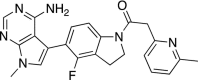 |
>10,000 | 100 | ||||||
| 1 | 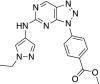 |
47. 6 | 119.3 | 24% | 93% | 14% | 37% | 0.65 | 16.0 |
| 2 | 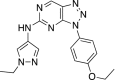 |
18.6 | 39.9 | <10% | 99% | <10% | 20% | 0.31 | 15.14 |
| 3 | 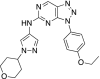 |
44.5 | 25% | ||||||
| 4 | 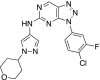 |
25.7 | <10% | 96% | <10% | 18% | 0.05 | 2.19 | |
| 5 | 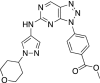 |
17.2 | <10% | 96% | <10% | 22% | 0.20 | 10.47 | |
| 6 | 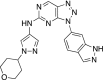 |
20.5 | 35% | ||||||
| 7 | 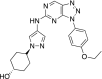 |
22.4 | 24% | ||||||
| 8 | 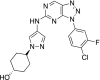 |
21.1 | <10% | 98% | <10% | 15% | 0.10 | 4.68 | |
| 9 | 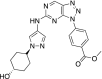 |
46.4 | 14% | 95% | <10% | 24% | 0.17 | 2.29 | |
All data are means of two independent experiments.
Compounds with PAMPA <10 × 10–6 cm/s are classified to have low permeability and compounds with a PAMPA >10 × 10–6 cm/s are classified as having high permeability.
