Abstract
ABO-incompatible liver transplants (ABO-ILT) have re-emerged as an alternate option for select patients awaiting transplant. However, treatment protocols for children undergoing deceased donor ABO-ILT are not standardized. We implemented a novel immunosuppression (IS) protocol for children undergoing deceased donor ABO-ILT based on pre-transplant isohemagglutinin (IH) titers. Children with high pre-transplant IH titers (≥1:32) underwent an enhanced IS protocol including plasmapheresis, rituximab, IVIG, and mycophenolate, while children with IH titers ≤ 1:16 received steroids and tacrolimus. We retrospectively assessed our outcomes of ABO-ILT with ABO-compatible recipients of similar age and diagnosis over a 2-year period. Ten children with median age of 8.9 months underwent ABO-ILT, 4/10 patients underwent enhanced IS due to high IH titers. Rates of complications (rejection, infections, biliary and vascular) at both 1 year and up to 3 years post-transplant were comparable between the groups. Patients with ABO-ILT had good graft function with 100% survival at a median follow up of 3.3 years. In conclusion, immunosuppression tailored to pre-transplant IH titers in pediatric deceased donor ABO-ILT is feasible and can achieve outcomes similar to ABO-CLT at 1 and 3 years post-transplantation.
Keywords: pediatric, ABO-incompatible, liver transplant, plasmapheresis, immunosuppression, isohemagglutinin, deceased donor
Background
Waitlist mortality in children awaiting liver transplantation (LT) remains high, with rates of 12–15% in patients < 2 years of age 1–3. To improve on this, it is critical for pediatric liver transplant centers to find alternate strategies to mitigate this risk of waitlist mortality by increasing donor availability and effective organ allocation 4. Interest in ABO-incompatible liver transplantation (ABO-ILT) has recently been renewed for select patients to broaden the donor pool and result in timelier LT 5,6. There is evolving evidence that complications historically associated with this procedure such as biliary, vascular, and early graft failure can be minimized with improved treatment protocols based on immunological monitoring prior to LT 7–10. Though recent studies report improved outcomes with ABO-ILT in younger children 6,11–13, immunological monitoring prior to ABO-ILT is lacking and treatment protocols for pediatric deceased donor transplantation are not standardized.
Isohemagglutinins (IH) are preformed IgM antibodies in circulation which can react to major blood group antigens A, B, and O on vascular endothelium in ABO-incompatible allografts. These antigen-antibody interactions can lead to complement activation, inflammation, and necrosis of the allograft resulting in early graft failure 14. In liver transplantation, this can further cause vascular and biliary tract complications 8. To combat these preformed antibodies, countries outside the US, where more than 10% of all living donor LT’s are ABO-incompatible, have incorporated desensitization protocols based on pre-transplant IH using rituximab and plasma exchange with excellent post-transplant survival rates 7,15–17.
Such treatment protocols are not directly applicable to deceased donor pediatric ABO-ILT, however, due to the unpredictable timing of the transplant operation. Consequently, we developed and implemented a novel ABO-ILT management protocol for pediatric deceased donor LT based on pre-transplant IH levels in a high volume center. The purpose of this protocol was to provide simplified and standardized evaluation, listing, and tailored management of pediatric ABO-ILT based on pre-transplant IH titers. In addition, we compared post-transplant outcomes of deceased donor ABO-ILT patients to ABO-compatible liver transplantation (ABO-CLT) during the same period at our institution.
Methods
Patient selection
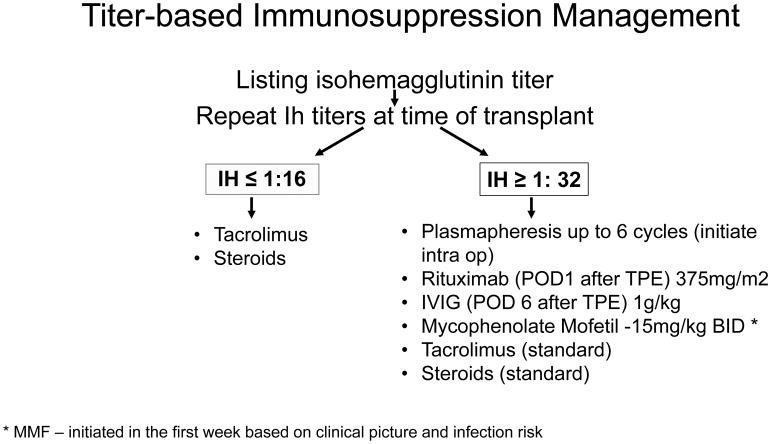
We developed an ABO-ILT management protocol for deceased donor LT based on pre-transplant IH titers. All pediatric recipients were listed under United Network for Organ Sharing (UNOS) criteria and a single transplant surgery team at Texas Children’s Hospital performed all transplants. Patients less than 2 years of age with 30 or more PELD/MELD points were listed with the option to receive ABO-ILT, as this is the lowest score at which UNOS allows ABO-ILT listing. We had one exception (patient #10) who was 9 years old at the time of transplant who received an ABO-ILT due to medical acuity. We obtained IgM anti-A/B IH titers at the time of listing for LT and again repeated at the time of transplant. Based on the highest titer to the relevant donor antigen, we divided patients into one of two groups. Patients with high IH titers (≥ 1:32) underwent enhanced IS protocol and low titers (≤ 1:16) received the standard IS protocol identical to our ABO-CLT patients (Figure 1) 7,15.
Figure 1.
Titer-based immunosuppression management: Flow diagram of IS protocol based on IH titers pre liver transplantation for children undergoing deceased donor liver transplantation. Children with IH ≤ 1:16 received standard IS while children with titers ≥ 1: 32 received enhanced IS.
Isohemagglutinin monitoring and immunosuppression protocol development
When a patient with high titers of ≥ 1:32 to the relevant antigen (based on the donor blood type) received a donor offer for ABO-ILT, the protocol was initiated in the pediatric intensive care unit (PICU). We initiated two plasma volume centrifugation based plasmapheresis (TPE), pre-operatively or intraoperatively using plasma compatible with recipient and donor. Post-transplantation, we administered 1.3 plasma volume exchange using plasma compatible with recipient and donor daily for up to 6 sessions or until the relevant IH titer was undetectable, whichever came first. We considered the trend of IH to be important and the goal of TPE was to reduce levels to 1:2 or less. Patients also received CD20 monoclonal antibody rituximab (375 mg/m2) after the 1st session of TPE and high dose intravenous immunoglobulin (IVIG, 1 gram/kg) after the last session of TPE. An anti-metabolite drug such as mycophenolate mofetil (15mg/kg/dose BID) was initiated based on clinical picture, age, and acute infection risk, in the first week post-transplant. ABO-ILT recipients with relevant IH titer ≤ 1:16 received our standard immunosuppression regimen identical to the ABO-CLT group. All ABO-ILT and ABO-CLT patients received intravenous methylprednisone (20mg/kg; maximum dose 1 gram) intraoperatively at the time of anastomosis followed by prednisone taper. Patients in both the groups received oral tacrolimus within a day of LT using our standard twice-daily dosing regimen, adjusted by trough measurements to target 10–12 ng/ml in the initial weeks after LT.
Following LT, IH titers were monitored daily in the ABO-ILT group for the first week, weekly for 3 weeks, monthly for 3 months, and then with annual labs. Additional IH panels were sent if there was suspicion for possible allograft rejection. When required, children who underwent ABO-ILT received plasma and platelet products compatible to both recipient and donor blood types (Supplementary Table 1).
Data collection and study design
The institutional review board at Baylor College of Medicine approved this study. From September 2013 to August 2015, 69 pediatric patients < 18 years of age underwent 72 deceased donor pediatric liver transplants at Texas Children’s Hospital. Among them, 10 patients underwent primary ABO-ILT. We compared post-transplant outcomes of children undergoing primary ABO-ILT (n=10) with ABO-CLT during the same period (n=19). Children of similar age and indication for transplantation were included for final analysis in our comparison ABO-CLT cohort. We calculated wait list times based on UNOS data for each patient including initial listing date and date of eligibility to receive ABO-ILT. We retrospectively obtained demographic characteristics, indication for LT, relevant surgical details, IH titers and hospital length of stay post-LT from the electronic medical record for all study patients. Post-operative complications (biliary, infection, vascular, rejection), as well as short (1 year) and longer-term (median 3.3 years) patient and graft survival were examined.
We excluded patients receiving re-transplantation and multi-visceral transplantation from analysis. Per our protocol, children with malignancy were excluded from receiving ABO-ILT. We defined infectious complications as signs and symptoms of infections, with positive microbiological cultures and/or molecular diagnostics, requiring hospital management. In addition, we noted biliary strictures requiring intervention and evaluated by percutaneous transhepatic cholangiography (PTC) or endoscopic retrograde cholangiopancreatography (ERCP) in patients with biochemical and ultrasound imaging abnormalities. Rejection episodes were defined as liver biopsy proven allograft rejection, requiring treatment with corticosteroids and increase in tacrolimus to maintain higher trough levels. We suspected antibody mediated rejection (AMR) in patients with elevated IH titers in conjunction with worsening liver function and suggestive histology and/or C4d immunofluorescence. We obtained data on vascular complications, including hepatic arterial thrombosis, and portal or hepatic venous thrombosis by radiographic reports.
Statistical analysis
We used STATA13 (StataCorp LP, College Station, TX) for data analysis. A p-value of <0.05 was considered statistically significant for all analyses. We summarized demographics, clinical characteristics, and complications 1 and 3 years post-transplantation using frequency with percentage and median with 25th and 75th percentiles. We compared patient characteristics and post-transplant complications in ABO-CLT and ABO-ILT groups using Fisher’s exact test or Wilcoxon rank sum test. Time to complications were compared between groups using log rank test and presented using Kaplan-Meier curves. Isohemagglutinin titers were graphed over time post-transplantation for ABO-ILT patients.
Results
Ten children received an ABO-ILT over the 24-month study period, with a median age of 8.9 months (range 5.1 months to 9.3 years). All patients were under 24 months of age except for a 9-year-old patient with acute liver failure. Five patients (50%) were female. The most common indication for LT was biliary atresia (BA) (60%). 19 ABO-CLT recipients with similar age, indication for LT, and medical acuity who underwent LT during the same period were included in the comparison group. Patients in both groups were listed with a Pediatric End-Stage Liver Disease (PELD) score of greater than or equal to 30 reflecting high medical acuity. Patient characteristics for both the groups are comparable and shown in Table 1.
Table 1.
Comparison between ABO-CLT and ABO-ILT recipients
| Characteristics* | ABO CLT | ABO ILT | p-value |
|---|---|---|---|
| N=19 | N=10 | ||
| Male, N (%) | 10 (53%) | 5 (50%) | 0.99 |
| Age (months), median (IQR) | 13.5 (8.2,20.6) | 8.9 (7.4,11.1) | 0.15 |
| Race, N (%) | 0.51 | ||
| African-American | 3 (16%) | 3 (30%) | |
| Caucasian | 4 (21%) | 0 | |
| Hispanic | 5 (26%) | 3 (30%) | |
| Unknown | 2 (11%) | 0 | |
| White: Other | 5 (26%) | 4 (40%) | |
| Diagnosis, N (%) | 0.48 | ||
| Biliary Atresia | 12 (63%) | 6 (60%) | |
| Cryptogenic Cirrhosis | 1 (5%) | 2 (20%) | |
| Fulminant Liver failure | 1 (5%) | 1 (10%) | |
| Metabolic | 5 (26%) | 1 (10%) | |
| PELD | 0.50 | ||
| ≥ 30 points | 11 (58%) | 4 (40%) | |
| Status 1A | 1 (5%) | 1 (10%) | |
| Status 1 B | 7 (37%) | 5 (50%) | |
| Graft type at transplant | 0.13 | ||
| Whole organ | 14 (74%) | 10 (100%) | |
| Left lateral segment, split | 5 (26%) | 0 | |
Compared using Fisher’s exact test or Wilcoxon rank sum test
Pre-transplant management
Three of ten ABO-ILT recipients had an initial IH titer against the donor blood type of ≥ 1:32. Peak pre-LT antibody titer in our cohort was 1:512 in an 8-month-old infant with BA (patient # 7). One additional child (patient #8) received enhanced IS protocol despite titer of 1:16 because she had received TPE pre-transplantation due to uncontrolled coagulopathy. Though her titer at the time of transplant was below our cut off, there was concern that it may not adequately reflect her humoral functional capacity due removal of circulating antibody by TPE with AB plasma prior to day of transplant. None of the patients in our group had TPE catheter related complications. Blood group types and IH titers for ABO-ILT group along with details of anastomosis is shown in Table 2.
Table 2.
ABO-ILT patients, transplant characteristics
| Patient No. | Gender/Age (Months) | Indication for transplant+ | ABO of Recipient | ABO of Donor | Pre-op Anti-ABO IgM | Graft type & Anastomosis* | TPE |
|---|---|---|---|---|---|---|---|
| 1 | F/9 | BA | O | A1 | Not available | Whole, RnY | No |
| 2 | M/5 | Cryptogenic cirrhosis | O | A | Not available | Whole, duct to duct, | No |
| 3 | F/19 | BA | A | AB | 1:2 | Whole, RnY | No |
| 4 | M/7 | CPS-1 | O | B | 1:8 | Whole, duct to duct | No |
| 5 | M/11 | BA | O | A1 | 1:2 | Whole, RnY | No |
| 6 | M/8 | BA | O | A1 | 1:8 | Whole RnY | No |
| 7 | F/8 | BA | O | A | 1:512 | Whole, RnY | yes |
| 8** | F/7 | BA | O | B | 1:16 | Whole, RnY | yes |
| 9 | F/7 | Cryptogenic cirrhosis | O | A1 | 1:64 | Whole, RnY | yes |
| 10 | M/111 | Fulminant liver failure | A | B | 1:64 | Whole, duct to duct | yes |
BA: Biliary Atresia, CPS-1: Carbamoyl phosphate synthetase deficiency 1
Duct to duct: Choledochocholedochostomy without t-tube, R n Y: Roux en Y hepaticojejunostomy
Patient had received TPE pre-transplantation for coagulopathy and hence received additional TPE post-LT due to concerns that her titers might have been falsely low due to removal of circulating antibody by TPE with AB plasma prior to day of transplant
In our cohort of patients, age at transplant was not associated with the presence or absence of antibody to blood group antigens, as patients under 1 year of age had high IH titers pre-transplantation (Spearman r = -.219, p=0.6).
Median total wait time from listing to transplantation was 75.5 (IQR, 50, 158) days for ABO-ILT and 95 days (IQR 51, 244) for ABO-CLT (p=0.313). The wait list time for already listed patients after opening to accept ABO-ILT was 46 (IQR 10, 81) days. Median length of stay post-transplant was comparable between the groups.
Changes in isohemagglutinin titers post ABO-ILT
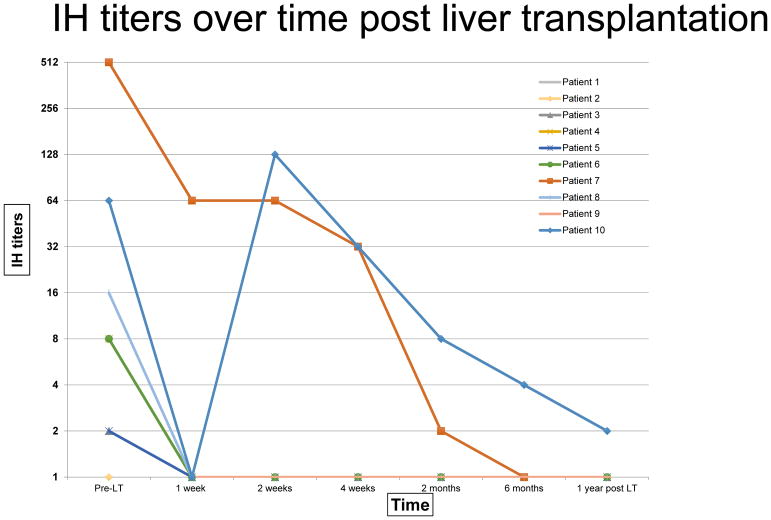
We monitored Isohemagglutinin titers per protocol after transplant. Patient #10 had a rebound IH anti-B titer of 1:128 on week 2, however immediate follow up the next day was 1:64, hence no alteration was made to the IS management. At a median of 2.8 years post ABO-ILT, relevant IH titers remains < 1:2 for all patients. Figure 2 shows the trend of IH titers pre and post ABO-ILT.
Figure 2.
IH titers over time post liver transplantation: Trend lines show individual patients with high and low titers who underwent ABO-ILT with tailored IS management and decline of IH over time post transplantation.
Complications post-transplant
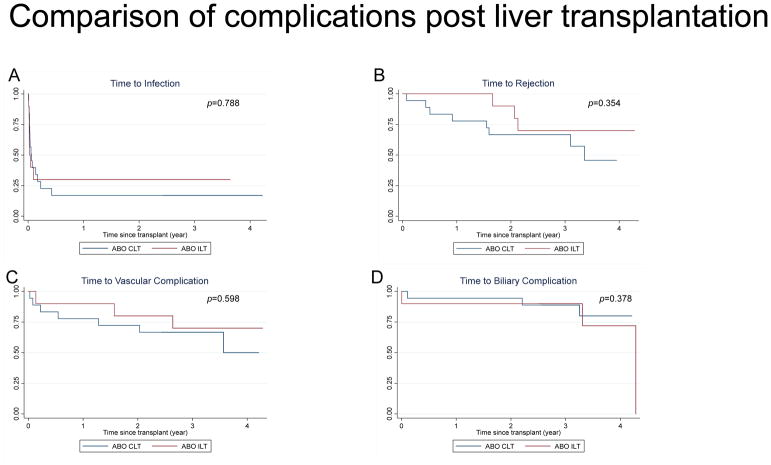
We compared between the patient groups rate of infections, acute rejection of allograft, biliary, and vascular complications in the first year after LT (Table 3) and in long term follow up with median time of 3.3 years (Table 4 and Figure 3). Patient #4 (donor blood type B, recipient blood type O, pre-LT IH 1:8) and patient #10 (donor blood type B, recipient blood type A, pre-LT IH 1:64) developed anastomotic biliary strictures 10–14 days post-transplant, which were diagnosed after rising conjugated bilirubin and gamma-glutamyl transferase. Both patients underwent ERCP with stent placement with resolution of symptoms. One patient in ABO-CLT group developed biliary stricture after early hepatic artery thrombosis and was re-transplanted for the same 2.5 years after initial transplant. While surveillance allograft biopsies are not performed at our institution, 3/10 patients had for-cause liver biopsies in ABO-ILT group and none demonstrated acute cellular or antibody-mediated rejection within the first year. Subsequently, beyond 1-year post-transplant, three patients in ABO-ILT group developed three episodes of acute cellular rejection. In the ABO-CLT group, three patients developed seven episodes of acute cellular rejection in the first year and five patients experienced eight episodes of acute cellular rejection beyond 1-year post-LT until the period of study follow up (Figure 3B).
Table 3.
Complications in the first year after LT
| Complications* | ABO CLT | ABO ILT | p-value |
|---|---|---|---|
| N=19 | N=10 | ||
| Infectious complications, N (%) | 15 (79%) | 7 (70%) | 0.665 |
| Acute cellular rejection, N (%) | 3 (15%) | 0 | 0.532 |
| Vascular complications, N (%) | 2 (11%) | 1 (10%) | 0.999 |
| Biliary complications, N (%) | 1 (5%) | 2 (20%) | 0.267 |
| Composite complications, N (%) | 16 (84%) | 8 (80%) | 0.999 |
| Length of stay (days), median (25, 75) | 27 (11, 62) | 32 (17, 87) | 0.463 |
| Graft loss, N (%) | 1 (5%) | 0 | 0.999 |
Fisher’s exact test to compare the groups
Table 4.
Complications after the first post-transplant year
| Complications* | ABO CLT | ABO ILT | p-value |
|---|---|---|---|
| N=18+ | N=10 | ||
| Infectious complications, N (%) | 8 (44%) | 6 (60%) | 0.695 |
| Acute cellular rejection, N (%) | 5 (28%) | 3 (30%) | 0.900 |
| Vascular complications, N (%) | 3 (17%) | 3 (30%) | 0.634 |
| Biliary complications, N (%) | 2 (11%) | 0 | 0.524 |
| Composite complications, N (%) | 11(61%) | 6(60%) | 0.999 |
| Follow up time, Median (min, max) | 3.3 (2.3, 4.2) | 3.4 (2.5, 4.3) | 0.748 |
Fisher’s exact test to compare the groups
One patient removed in ABO-CLT group due to death within first year post-transplant
Figure 3.
Comparison of complications post liver transplantation: Kaplan-Meier curves represent post-transplant complications of infection (A), rejection (B), vascular (C) and biliary (D) complications over time (in years) after transplant. ABO-ILT group is represented in red lines and ABO-CLT groups in blue lines.
There were no significant differences in the rate or severity of infection between the groups. We observed bacterial infections in 70% and viral infections in 50% in the ABO-ILT group, which was comparable to 64% and 57%, respectively, in the ABO-CLT group. Gram-negative rods and adenovirus were the most commonly isolated organisms in both groups in the first year post-LT. Beyond 1-year post-LT, 60% of ABO-ILT and 44% ABO-CLT patients had infections. Respiratory viral infections such as Influenza and RSV, as well as Clostridium difficile diarrhea were the most common identified pathogens. Within the ABO-ILT group, 7/10 patients had infectious complications in the first year post-transplant, out of which only 2/7 had received additional IS due to high pre-transplant IH titers. None of the patients in the cohort developed post-transplant lymphoproliferative disease. In the ABO-CLT group, two patients had graft loss due to hepatic artery thrombosis requiring re-transplantation (one early on post-op day 3 and the other 2.5 years after initial transplant). There was 100% graft survival in ABO-ILT group. In the ABO-ILT group, one patient had portal vein stenosis 2 months post-transplant requiring repeated balloon dilatation and stenting with subsequent resolution. Two patients in each group had portal vein thrombosis requiring recanalization by interventional radiology beyond the first year post-LT. There was one death immediately post-LT in ABO-CLT group due to primary non-function of the graft, but 100% survival in ABO-ILT group.
Discussion
In this study of pediatric deceased donor ABO-incompatible liver transplant recipients, we demonstrate that IH titer-based tailored immunosuppression is feasible and leads to outcomes comparable to ABO-compatible transplantation. Increasing organ shortage and resultant high waitlist mortality demands expanding the donor pool to cross the blood group barrier for deceased donor liver transplantation 4. ABO-ILT has shown mixed results with some groups reporting higher graft loss, while some centers with aggressive immunosuppression reporting acceptable graft and patient survival, albeit with increased rejection and infection rates 12,18–21. A recent Japanese study of living donor LT showed higher graft loss with ABO- incompatible grafts compared to ABO-compatible or identical allografts at long term, 20 year follow up. However, even in this large series, recipients <2 years of age at the time of LT had significantly better outcomes compared to recipients ≥2 (81.2% versus 50.2%) 22. Our study adds to the current limited literature in ABO-incompatible deceased donor liver transplantation demonstrating good patient and graft survival similar to other pediatric analyses 6,12,21.
Small single center studies in children undergoing living-related donor transplantation suggest elevation of IH titers after transplantation may be a predictive risk factor for increased mortality and morbidity and the reduction of titers of anti-donor blood-type antibodies using TPE and rituximab can achieve favorable outcomes 7,15,23. Including rituximab is critical, in combination with TPE to reduce complement-dependent and antibody-dependent cellular cytotoxicity induced by anti-donor ABO antibodies via B-cells 7,8,15,24,25. Thus far, there is no IS protocol for deceased donor ABO-ILT and the living donor management protocols are not directly applicable as there is limited time for pre-transplant immunomodulation. We have successfully implemented an IH titer-based protocol that gives a practical and simplified approach for targeted IS alteration in patients undergoing ABO-ILT with high antibody titers. This approach spares patients with low titers (≤1:16) from higher IS administration and procedures such as TPE.
Contrary to the assumption that children under 2 years of age have immature immune systems and they seldom mount antibodies against ABO, in our experience we found that infants with end-stage liver disease can have high IH titers of 1:512 26. As IH are IgM antibodies, there is no chance that they are from maternal passive transfer and are thus truly reflective of humoral capacity. This reinforces the importance of pre-transplant IH monitoring across all age groups, including infants. Post-transplantation, IH is an important part of assessing the patients overall clinical picture and clinical decision making in concert with other abnormalities, like increase in transaminases, which would prompt rapid assessment and treatment. Patients in our cohort with low titers who underwent standard IS showed persistently low titers at median of 2.8 years post-transplant, indicating immunological accommodation for the donor graft.
ABO-ILT has been associated with increased rejection and infection rates in both adult and pediatric populations. The strength of our data is that the rate of allograft rejection post-LT was comparable in ABO-ILT group to the ABO-CLT group. This we attribute to our unique protocol, as we monitor IH titers pre-transplant and alter IS selectively for patients with high IH titers in order to prevent development of complications such as AMR after LT. Our approach is in contrast to the study by Heffron et al where IS was altered after the rise of IH titers and complications post-transplant12. Interestingly, within the ABO-ILT group, 5/7 patients who developed infections in the first year after transplant had low IH titers and received only standard IS with steroids and tacrolimus. Overall rates and severity of infection were comparable to ABO-CLT group in our cohort. Compared to the ABO-CLT group, the graft and patient outcomes at 1 year and 3 years were similar to the ABO-ILT group which furthers the potential utility of pediatric ABO-ILT transplants as alluded by a meta-analysis by Wu et al 21.
While we did not find any statistically significant difference in the wait list times between the groups, there was a trend towards shorter wait list times in the ABO-ILT group. ABO-ILT patients were transplanted at a median of 46 days following eligibility for ABO-ILT, which according to present OPTN policy is to have a minimum of 30 PELD points. It is conceivable that a policy change to allow earlier eligibility for ABO-ILT could be an effective measure to shorten wait list times. While it is not part of the protocol to select only whole organ versus split allografts for ABO-ILT, coincidentally in this study all the patients in the ABO-I group received a whole organ allograft, as is the case in about 75% of patients transplanted at our center.
There are several limitations to this study. We tested for IgM antibodies but additional testing of IgG antibodies to donor antigens may be important and the significance of such monitoring is currently unknown. Though our institution is a high volume pediatric LT center, we have limited sample size for the study especially in children older than 2 years of age who underwent ABO-ILT, limiting statistical power and analysis. An additional limitation is lack of long-term follow up to evaluate graft outcomes in ABO-ILT group. We will continue to monitor IH titers annually and with any for-cause liver biopsies in these patients.
In conclusion, transplanting across blood group barriers can be successful in young patients, under the age of two with careful management, even with high pre-transplant IH titers. Our experience adds evidence for this approach and suggests consideration of titer based management of ABO-ILT in patients who otherwise might not receive a donor organ in a timely fashion on the LT waiting list. Future multicenter studies and longer follow up periods are necessary to routinely implement this IS protocol for ABO-ILT.
Supplementary Material
Supplementary Table 1: Blood products administered after ABO-ILT
Acknowledgments
Grants and financial support: Cade R Alpard Foundation for Pediatric Liver Disease. KRM was supported by a grant from the National Institutes of Health (T 32DK007664)
Abbreviations
- ABO-ILT
ABO-incompatible liver transplantation
- ABO-CLT
ABO-compatible liver transplantation
- IH
isohemagglutinin
- LT
liver transplantation
- IVIG
Intravenous Immunoglobulin
- TPE
therapeutic plasma exchange
- UNOS
United Network for Organ Sharing
- PELD
pediatric end-stage liver disease
- MELD
model for end-stage liver disease
- BA
biliary atresia
- CPS-1
carbamoyl phosphate synthetase deficiency 1
- d to d
choledochocholedochostomy without t-tube
- R n Y
Roux en Y hepaticojejunostomy
- ERCP
endoscopic retrograde cholangiopancreatography
- AMR
antibody mediated rejection
Footnotes
CONFLICT OF INTEREST
The listed authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
AUTHORS’ CONTRIBUTIONS
All authors: Participated in the concept/design, implementation of the study protocol, data interpretation, manuscript preparation and/or critical revision of article.
Danielle Guffey, and Dr. Charles Minard: Participated in data analysis and revision of the manuscript. All authors have approved the article.
References
- 1.Leung DH, Narang A, Minard CG, Hiremath G, Goss JA, Shepherd R. A 10-Year united network for organ sharing review of mortality and risk factors in young children awaiting liver transplantation. Liver Transpl. 2016;22(11):1584–1592. doi: 10.1002/lt.24605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pugliese R, Fonseca EA, Porta G, et al. Ascites and serum sodium are markers of increased waiting list mortality in children with chronic liver failure. Hepatology. 2014;59(5):1964–1971. doi: 10.1002/hep.26776. [DOI] [PubMed] [Google Scholar]
- 3.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2013 Annual Data Report: liver. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(Suppl 2):1–28. doi: 10.1111/ajt.13197. [DOI] [PubMed] [Google Scholar]
- 4.Hsu EK, Shaffer ML, Gao L, et al. Analysis of Liver Offers to Pediatric Candidates on the Transplant Wait List. Gastroenterology. 2017;153(4):988–995. doi: 10.1053/j.gastro.2017.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raut V, Uemoto S. Management of ABO-incompatible living-donor liver transplantation: past and present trends. Surg Today. 2011;41(3):317–322. doi: 10.1007/s00595-010-4437-3. [DOI] [PubMed] [Google Scholar]
- 6.Rana A, Kueht ML, Nicholas SK, et al. Pediatric Liver Transplantation Across the ABO Blood Group Barrier: Is It an Obstacle in the Modern Era? Journal of the American College of Surgeons. 2016;222(4):681–689. doi: 10.1016/j.jamcollsurg.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 7.Ikegami T, Taketomi A, Soejima Y, et al. Rituximab, IVIG, and plasma exchange without graft local infusion treatment: a new protocol in ABO incompatible living donor liver transplantation. Transplantation. 2009;88(3):303–307. doi: 10.1097/TP.0b013e3181adcae6. [DOI] [PubMed] [Google Scholar]
- 8.Song GW, Lee SG, Hwang S, et al. Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol. 2014;61(3):575–582. doi: 10.1016/j.jhep.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 9.Urbani L, Mazzoni A, Bianco I, et al. The role of immunomodulation in ABO-incompatible adult liver transplant recipients. J Clin Apher. 2008;23(2):55–62. doi: 10.1002/jca.20156. [DOI] [PubMed] [Google Scholar]
- 10.Kim JD, Choi DL, Han YS. Fourteen successful consecutive cases of ABO-incompatible living donor liver transplantation: new simplified intravenous immunoglobulin protocol without local infusion therapy. Transplantation proceedings. 2014;46(3):754–757. doi: 10.1016/j.transproceed.2013.11.100. [DOI] [PubMed] [Google Scholar]
- 11.Stewart ZA, Locke JE, Montgomery RA, Singer AL, Cameron AM, Segev DL. ABO-incompatible deceased donor liver transplantation in the United States: a national registry analysis. Liver Transpl. 2009;15(8):883–893. doi: 10.1002/lt.21723. [DOI] [PubMed] [Google Scholar]
- 12.Heffron T, Welch D, Pillen T, et al. Successful ABO-incompatible pediatric liver transplantation utilizing standard immunosuppression with selective postoperative plasmapheresis. Liver Transpl. 2006;12(6):972–978. doi: 10.1002/lt.20760. [DOI] [PubMed] [Google Scholar]
- 13.Gelas T, McKiernan PJ, Kelly DA, Mayer DA, Mirza DF, Sharif K. ABO-incompatible pediatric liver transplantation in very small recipients: Birmingham’s experience. Pediatric transplantation. 2011;15(7):706–711. doi: 10.1111/j.1399-3046.2011.01541.x. [DOI] [PubMed] [Google Scholar]
- 14.Demetris AJ, Jaffe R, Tzakis A, et al. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. The American journal of pathology. 1988;132(3):489–502. [PMC free article] [PubMed] [Google Scholar]
- 15.Kawagishi N, Satoh K, Enomoto Y, et al. New strategy for ABO-incompatible living donor liver transplantation with anti-CD20 antibody (rituximab) and plasma exchange. Transplantation proceedings. 2005;37(2):1205–1206. doi: 10.1016/j.transproceed.2004.12.114. [DOI] [PubMed] [Google Scholar]
- 16.Uchiyama H, Mano Y, Taketomi A, et al. Kinetics of anti-blood type isoagglutinin titers and B lymphocytes in ABO-incompatible living donor liver transplantation with rituximab and plasma exchange. Transplantation. 2011;92(10):1134–1139. doi: 10.1097/TP.0b013e318231e9f8. [DOI] [PubMed] [Google Scholar]
- 17.Soejima Y, Muto J, Matono R, et al. Strategic breakthrough in adult ABO-incompatible living donor liver transplantation: preliminary results of consecutive seven cases. Clin Transplant. 2013;27(2):227–231. doi: 10.1111/ctr.12060. [DOI] [PubMed] [Google Scholar]
- 18.Egawa H, Oike F, Buhler L, et al. Impact of recipient age on outcome of ABO-incompatible living-donor liver transplantation. Transplantation. 2004;77(3):403–411. doi: 10.1097/01.TP.0000110295.88926.5C. [DOI] [PubMed] [Google Scholar]
- 19.Imagawa DK, Noguchi K, Iwaki Y, Busuttil RW. Hyperacute rejection following ABO-compatible orthotopic liver transplantation--a case report. Transplantation. 1992;54(6):1114–1117. [PubMed] [Google Scholar]
- 20.Gugenheim J, Samuel D, Reynes M, Bismuth H. Liver transplantation across ABO blood group barriers. Lancet (London, England) 1990;336(8714):519–523. doi: 10.1016/0140-6736(90)92082-s. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Ye S, Xu X, Xie H, Zhou L, Zheng S. Recipient outcomes after ABO-incompatible liver transplantation: a systematic review and meta-analysis. PloS one. 2011;6(1):e16521. doi: 10.1371/journal.pone.0016521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasahara M, Umeshita K, Sakamoto S, et al. Living donor liver transplantation for biliary atresia: An analysis of 2085 cases in the registry of the Japanese Liver Transplantation Society. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2018;18(3):659–668. doi: 10.1111/ajt.14489. [DOI] [PubMed] [Google Scholar]
- 23.Ashizawa T, Matsuno N, Yokoyama T, et al. The role of plasmapheresis therapy for perioperative management in ABO-incompatible adult living donor liver transplantation. Transplantation proceedings. 2006;38(10):3629–3632. doi: 10.1016/j.transproceed.2006.10.122. [DOI] [PubMed] [Google Scholar]
- 24.Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(5 Pt 1):859–866. doi: 10.1111/j.1600-6143.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee SD, Kim SH, Kong SY, Kim YK, Park SJ. Kinetics of B, T, NK lymphocytes and isoagglutinin titers in ABO incompatible living donor liver transplantation using rituximab and basiliximab. Transplant immunology. 2015;32(1):29–34. doi: 10.1016/j.trim.2014.11.216. [DOI] [PubMed] [Google Scholar]
- 26.Fong SW, Qaqundah BY, Taylor WF. Developmental patterns of ABO isoagglutinins in normal children correlated with the effects of age, sex, and maternal isoagglutinins. Transfusion. 1974;14(6):551–559. doi: 10.1111/j.1537-2995.1974.tb04576.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Blood products administered after ABO-ILT