Abstract
Recent evidence indicates that the relationship between increased beta-amyloid (Aβ) deposition and functional task-activation can be characterized by a non-linear trajectory of change in functional activation (Foster et al., 2017), explaining mixed results in prior literature showing both increases and decreases in activation as a function of beta-amyloid burden in cognitively normal adults. Here we sought to replicate this nonlinear effect in the same sample using a different functional paradigm to test the generalizability of this phenomenon. Participants (N=68 healthy adults aged 49-94) underwent fMRI (0-, 2-, 3-, 4-back working memory task; WM) and 18F-Florbetapir PET scanning. A parametric WM load contrast was used as the dependent variable in a model with age, mean cortical Aβ, and Aβ2 as predictors. Results revealed that nonlinear amyloid (Aβ2) was a significant negative predictor of modulation of activation to WM load in two large inferior clusters: bilateral subcortical nuclei and bilateral lateral cerebellum. Individuals with slightly elevated Aβ burden evidenced greater modulation as compared to individuals with little or no Aβ burden, whereas individuals with the greatest Aβ burden evidenced lesser modulation as compared to individuals with slightly elevated Aβ. Increased modulation to WM load predicted better task accuracy and executive function measured outside the scanner. The current study provides further evidence for a dose-response, nonlinear relationship between increasing Aβ burden and alteration in brain activation in cognitively healthy adults, extending the existing evidence to dynamic range of activation to task difficulty, and reconciling seemingly discrepant effects of amyloid on brain function.
Keywords: Aging, Beta-amyloid, fMRI, preclinical Alzheimer’s Disease, nonlinear BOLD activation, cognition, N-Back
Introduction
The accumulation of beta-amyloid (Aβ) plaque in the brain is considered to be one of the earliest events in the pathogenesis of Alzheimer’s Disease (AD). Importantly, measurable amounts of Aβ protein are detectable 15-20 years before cognitive or clinical symptoms become visibly manifested (Dubois et al., 2016; Jack et al., 2010, 2013; Jansen et al., 2015; Rowe et al., 2010). The effects of Aβ on the local neural environment are complex, manifold, and incompletely understood (Palop & Mucke, 2010), but it is evident that the cellular signaling and firing of these neurons is altered with the presence of Aβ plaque (Sperling, Mormino, & Johnson, 2014). For this reason, the effects of Aβ burden on brain function in cognitively-normal adults have been widely studied, especially for episodic memory (Elman et al., 2014; Huijbers et al., 2014; Kennedy et al., 2012; Leal et al., 2017; Mormino et al., 2012; Oh et al., 2015; Oh, Steffener, Razlighi, Habeck, & Stern, 2016; Sperling et al., 2009). However, rather than coalescing into a coherent picture, the results are mixed with some studies reporting increases in activation and others decreases in activation with increasing Aβ.
We recently reported that a nonlinear relationship between Aβ burden and BOLD response to a cognitive task exists that can explain this discrepancy seen across studies in the literature (Foster, Kennedy, Horn, Hoagey, & Rodrigue, 2018). Specifically, in regions that deactivated in response to task difficulty, this nonlinear relationship demonstrated that at lower levels of Aβ burden there is increased activation (i.e., decreased de-activation) to difficult spatial judgments, whereas at highest levels of Aβ burden, there is decreased activation (i.e., increased de-activation) to difficulty (Foster et al., 2018). Furthermore, as the amyloid-activation association flipped, so did the nature of its effect on performance, whereby in individuals at the lower range of clinically relevant Aβ burden, greater de-activation was associated with better performance, but in individuals with even greater Aβ, greater de-activation was associated with poorer performance, suggesting that activation altered by Aβ is detrimental to cognition.
The goal of the current study is to test the generalizability of this phenomenon of nonlinearity of brain function to differing levels of Aβ deposition in cognitively normal older adults with another task paradigm to replicate the findings of Foster et al., 2018. Dynamic range of task-activation appears to be evidencing as a reliable marker of cognitive aging and brain functional integrity (Garrett, Kovacevic, Mcintosh, & Grady, 2013; Garrett, Lindenberger, Hoge, & Gauthier, 2017; Hakun & Johnson, 2017; Kennedy, Boylan, Rieck, Foster, & Rodrigue, 2017; Kennedy et al., 2015; Persson, Lustig, Nelson, & Reuter-Lorenz, 2007; Rieck, Rodrigue, Boylan, & Kennedy, 2017) and thus, we chose to examine the effects of Aβ on the ability to modulate BOLD activity to parametrically increasing working memory (WM) load in the same sample of participants used in Foster et al., (2018). Based on the prior findings, it is possible that the nonlinear increase-to-decrease of activity may reflect a fundamental property of the brain’s response to various levels of Aβ pathology and thus, should be task-independent in its manifestation. Indeed, a nonlinear function has been suggested even in task-free fMRI (Schultz et al., 2017). We hypothesize that the relation of BOLD modulation to Aβ levels will be nonlinear, where at the lowest levels of Aβ deposition, modulation is increased, but at higher levels of Aβ burden, modulation will decrease in a dose-response fashion with increasing beta-amyloid levels. We further hypothesize that this reduced modulation to difficulty is detrimental in nature, and thus, will be associated with poorer cognitive performance.
Methods
Participants
Participants included 68 healthy, cognitively normal adults (mean age = 68.59 ± 10.73; age range 49–94 years) who were drawn from a larger study of 181, of whom 72 had both fMRI and amyloid-PET data. Eighteen participants were deemed to have elevated Aβ using a standardized uptake value ratio (SUVR) cutoff of 1.11 (Clark et al., 2011; see Table 1). All participants were recruited from the Dallas-Fort Worth metroplex and screened to ensure they were right-handed, fluent English speakers, with normal or corrected-to-normal vision. When required, MRI-compatible glasses were used during scanning. Participants were also screened against a history of metabolic, neurologic or psychiatric conditions, head trauma, drug or alcohol problems, cardiovascular disease, depression (Center for Epidemiological Study - Depression < 16; Radloff, 1977), and to be cognitively intact (Mini Mental State Exam ≥ 26; Folstein, Folstein, & McHugh, 1975). There were no significant Aβ (high vs low) group differences on any measure (p’s > .150) other than age (t(45.43) = −3.17, p = .003). Twenty-five participants in the current sample self-reported a diagnosis of hypertension and there was no relationship between dichotomous Aβ and hypertension status variables (36% hypertensives in low- vs. 39% in high-Aβ, χ2 = 0.048, p = .828).
Table 1.
Participant Demographics and Task Performance
| Low Aβ | High Aβ | Total | |
|---|---|---|---|
| N (% Female) | 50 (64.00) | 18 (44.44) | 68 (58.82) |
| Mean Age (SD) | 66.62 (11.12)* | 74.06 (7.40) | 68.59 (10.73) |
| Mean Education (SD) | 15.30 (2.68) | 16.33 (2.50) | 15.57 (2.66) |
| Mean MMSE (SD) | 28.84 (0.82) | 28.83 (0.71) | 28.84 (0.78) |
| Mean CESD (SD) | 3.88 (3.78) | 3.44 (3.71) | 3.77 (3.74) |
| fMRI Task Accuracy | |||
| 0-Back (SD) | 94.00 (10.65) | 93.80 (9.90) | 98.33 (10.38) |
| 2-Back (SD) | 83.70 (11.92) | 83.75 (10.42) | 83.17 (11.50) |
| 3-Back (SD) | 76.75 (8.68) | 72.82 (5.83) | 75.71 (8.17) |
| 4-Back (SD) | 74.77 (7.84) | 73.01 (5.88) | 74.30 (7.37) |
| fMRI Task RT (sec) | |||
| 0-Back (SD) | 0.68 (0.14) | 0.63 (0.07) | 0.66 (0.13) |
| 2-Back (SD) | 0.90 (0.20) | 0.82 (0.16) | 0.88 (0.19) |
| 3-Back (SD) | 1.01 (0.23) | 0.90 (0.18) | 0.98 (0.22) |
| 4-Back (SD) | 1.00 (0.25) | 0.88 (0.17) | 0.97 (0.23) |
| EF Composite (SD) | 0.04 (0.56) | −0.09 (0.56) | 0.00 (0.56) |
| WM Composite (SD) | 0.02 (0.83) | −0.06 (0.72) | 0.00 (0.80) |
Note: Low Aβ – less than 1.11 standardized uptake value ratio; High Aβ – greater than or equal to 1.11 standardized uptake value ratio. MMSE - Mini Mental State Exam; CESD – Center for Epidemiologic Study-Depression; Accuracy reported as mean percent accuracy; Response time (RT) reported as a mean of medians such that a median RT is taken across all trials in a given condition for each subject and averaged across subjects; SD – standard deviation; sec – seconds; EF – executive function; WM – working memory; both are averages of standardized test measures reported as z-scores;
p < .05.
Four of the initial 72 participants were excluded from analysis due to MRI acquisition issues (two for structural image acquisition and two for functional image acquisition issues). The four excluded participants did not differ significantly from the included participants, respectively, in age [t(3.38) = −1.13, p = .333; 62.50 ± 10.47 SD vs 68.59 ± 10.73], education [t(3.28) = −0.70, p = 0.53; 14.50 ± 3.00 vs 15.57 ± 2.66], MMSE [t(3.68) = −1.11, p = 0.33; 28.50 ± 0.58 vs 28.84 ± 0.78], or CESD [t(3.40) = 0.13, p = 0.91; 4.00 ± 3.56 vs 3.76 ± 3.74]. One excluded participant was Aβ-positive.
Neuroimaging Protocol
PET Acquisition.
Participants were scanned on a single Siemens ECAT HR PET scanner at UT Southwestern Medical Center. All participants were injected with 370 MBq (10 mCi) of 18F-Florbetapir (Avid Radiopharmaceuticals/Eli Lilly). Approximately 30 minutes post-injection, participants were placed on the imaging table and foam wedges were used to secure the participant’s head. A 2-minute scout was acquired to ensure the brain was within the field of view. Fifty minutes post-injection, an internal rod source transmission scan was acquired for 7 minutes immediately followed by a 2-frame by 5 minutes each dynamic emission acquisition. The transmission image was reconstructed using back-projection with a 6-mm full-width at halfmaximum (FWHM) Gaussian filter. Emission images were processed by iterative reconstruction, 4 iterations and 16 subsets with a 3-mm FWHM ramp filter.
PET Data Processing.
Each participant’s PET scan was first registered to their T1-weighted MRI image with a rigid affine registration using Advanced Normalization Tools (ANTs) (Avants, Tustison, Song, & Gee, 2009) scripts and visually inspected for registration quality. Freesurfer (Fischl, 2012) parcellations of interest that correspond to the traditionally used 7 ROIs for Aβ deposition (i.e., anterior cingulate, posterior cingulate, precuneus, lateral temporal, lateral parietal, middle frontal, and inferior frontal) were also registered to each subject’s T1 image. Using methods outlined in Rodrigue et al., (2012), uptake counts were extracted from each ROI and normalized to whole cerebellar counts to yield Aβ SUVRs for each ROI. All ROIs were averaged to form a mean cortical Aβ index. Mean cortical Aβ was also selected as our independent variable because Aβ effects on BOLD modulation were not hypothesized to occur in spatially overlapping regions.
MRI Acquisition.
Participants were scanned on a single Philips Achieva 3T whole-body scanner equipped with a 32-channel head coil. High-resolution anatomical images were collected with a Tl-weighted MP-RAGE sequence with the following parameters: 160 sagittal slices, 1 × 1 × 1 mm3 voxels; FOV = 256 mm × 204 mm × 160 mm, FOV = 256 mm, TE = 3.8 ms, TR = 8.3 ms, FA = 12°. Blood Oxygenation Level Dependent (BOLD) fMRI data were acquired using a T2*-weighted echo planar imaging sequence in 29 interleaved axial slices parallel to AC-PC line: 64 × 64 matrix, 3.4 × 3.4 × 5 mm3 voxels, FOV = 220 mm × 145 mm × 220 mm, TE = 30 ms, TR = 1500 ms.
fMRI Data Processing.
Individual participant time-series data were preprocessed with Statistical Parametric Mapping 8 (SPM8; Wellcome Department of Cognitive Neurology, London, UK) according to a standard pipeline of procedures. Images were corrected for differences in slice-time acquisition, individual volumes were corrected for within-run participant movement, and images were normalized to a common MNI space and smoothed with an isotropic 8 mm3 FWHM Gaussian kernel, respectively. ArtRepair (Mazaika, Hoeft, Glover, & Reiss, 2009) was used to identify intensity and motion outlier volumes. For each participant, runs that had more than 15% outlier volumes (~30 volumes) with > 3% deviation from the mean in global intensity spikes or > 2 mm of motion displacement were excluded. Participants with more than one run flagged for excessive outlier volumes were excluded from the study entirely (n = 0 in this sample).
At the individual subject level, BOLD response to each condition (0-, 2-, 3-, and 4-back) was modeled in SPM as a block convolved with a canonical hemodynamic response function; six directions of motion-estimates for each volume generated from ArtRepair were also included as nuisance covariates. A linear parametric contrast [−2.25, −0.25, 0.75, and 1.75] was used at the individual subject level modeling the increase in working memory (WM) load across the four conditions.
Experimental Design and Statistical Analysis
fMRI n-back task.
In brief, participants performed blocks of 0-, 2-, 3-, and 4-back task conditions across 3 runs, each lasting approximately 6 minutes. Each run of data consisted of 8 blocks, including 2 blocks of each level of difficulty. The blocks were counterbalanced for difficulty within run. Before each block began, a 5-second cue indicated which type of n-back condition was about to begin: 0-, 2-, 3-, or 4-back, followed by 2 seconds of fixation prior to the presentation of stimuli (digits). For each trial, participants made a SAME/DIFFERENT response with their index (same) or middle (different) finger to indicate whether the digit presented was the same or different as n digits ago. Digits (“2 - 9”) were presented for 500 ms with a 2000 ms interstimulus interval using Psychopy v1.77.02 (Peirce, 2007, 2009) in a pseudorandom order. For a more detailed description of the task procedure see Kennedy, Boylan, Rieck, Foster, & Rodrigue (2017). To evaluate the behavioral effects of the n-back difficulty manipulation, age, and Aβ burden on the task performance, we ran two repeated-measures ANOVAs with the four working memory load conditions as a within-subject variable, age and A β as between-subjects variables, and all interactions as predictors of either accuracy or response time (RT).
fMRI Data Analysis.
A whole-brain voxel-wise linear regression was conducted with an intercept, mean-centered age at the time of fMRI, mean-centered cortical Aβ, and the squared term (i.e., quadratic) of mean cortical Aβ2 as independent variables in a model with task modulation [linear change in BOLD activation in response to WM load] as the dependent variable. All results were tested using t-tests for each effect and cluster-corrected to FWE p < .05 using SnPM with a height threshold of p < .005 and 5000 permutations (SnPM13; http://warwick.ac.uk/snpm) and using a gray matter mask created from a liberally thresholded average of the canonical grey matter mask in SPM8 and each subject’s grey matter mask. As the test of our primary hypothesis, that Aβ burden would relate to positive and/or negative modulation, we examined the quadratic effect of Aβ on modulation. To test for regions that showed a linear effect, but no significant quadratic effect, a mask was created from the quadratic effect using a height threshold of p < .05 with a zero-voxel cluster extent. This mask was used as an exclusion mask when investigating the linear effect, tested within the full model.
To yield the pattern of regions that modulated in response to increased WM load, we conducted a positive and negative test of the intercept. In this model, because each variable was mean-centered, tests of this effect represented an estimate of positive modulation and negative modulation at the mean of all other variables in the model.
To gauge the potential effects contributed by relevant covariates (APOEε4 status, brain size), three separate follow-up analyses (using the same SPM model specification) were also run controlling for APOE status, average gray matter thickness (estimated using Freesurfer and averaged across all gray matter parcels), and total gray matter volume (estimated using Freesurfer and summing over all gray matter parcels) after adjusting for manually traced intracranial volume (Raz et al., 2005). We re-estimated the same model (i.e., intercept, mean-centered age, mean-centered Aβ, and Aβ2) with gray matter volume or thickness entered as continuous covariates. While gray matter thickness and volume measures were available for all participants, only 64 of the 68 participants had APOEε4 genotype information (see Foster et al., 2017 for genotyping details). Therefore, to maximize sample size and power, including APOE as a covariate was conducted as a follow-up to the primary model. Again, re-estimating the same model (i.e., intercept, mean-centered age, mean centered Aβ, and Aβ2) but with the reduced number of individuals, APOEε4 status (ε4− and ε4+) was entered as a dichotomous covariate where ε4− individuals were the reference group. A total of 13 individuals were APOE ε4+ and five of these were classified as high-Aβ (31.25%) and 8 individuals were classified as low-Aβ (16.66%). Further, of the four individuals without genotype information, two were low-Aβ and two were high-Aβ.
Cognitive Measures
Prior to MRI scanning, participants underwent two days of cognitive testing in which a variety of cognitive measures were collected. For the purposes of the current study we utilized unit-weighted composite measures of working memory (WM) and executive function (EF) to investigate relationships between Aβ and modulation, and age. All tests were z-scored before calculating the unit-weighted composite. The WM composite consisted of number correct on Wechsler Adult Intelligence Scale – IV (WAIS - IV; Wechsler, 2008) Digit Span (forward, backward, and sequencing) and the Listening Span task (Salthouse, Babcock, & Shaw, 1991) subscores (simple span, absolute span, and total span). WM composite Cronbach’s α = .88.
The EF composite consisted of several subtests of the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001) including Verbal Fluency (subtests: category fluency – total average correct of animals and boys names; verbal fluency – average total correct for F, A, S), the Trail Making Test (subtests: trail switching adjusted for average time on trail numbers and trail letters), the Color Word Interference Test (subtests: color-word inhibition adjusted for average time on color naming and color reading; color-word inhibition switching adjusted for average time on color naming and color reading), and the Wisconsin Card Sorting Test (WCST; Heaton, Chelune, Talley, Kay, & Curtiss, 1993) subscores: number of categories completed, number of trials to complete first category, and number of times for a failure to maintain set. EF composite Cronbach’s α = .69.
The relationships between Aβ, modulation, and behavior (i.e., task accuracy, EF, and WM) were examined to better understand the nature of the individual differences on these three measures of cognitive performance. In each of the three multiple regression models we included age, as well as mean-centered modulation (i.e., modulation extracted from all significant voxels in the effect of interest using the eigenvariate function in SPM), mean-centered Aβ, and the interaction between modulation and Aβ as predictors of each behavioral outcome.
Results
fMRI n-back Behavioral Performance
There were significant effects of n-back load on accuracy (F(3,192) = 97.66, p < .001) and response time (F(3,192) = 110.54, p < .001), with poorer accuracy and slower RT with increasing load. There was also a significant effect of age on accuracy (F(1,64) = 8.80, p = .004) where increasing age was associated with decreasing accuracy. No other effects reached statistical significance, ps > .052. See Table 1 for task performance descriptive statistics.
fMRI Results – Effects of Age and Amyloid Burden on BOLD Modulation
A whole-brain voxel-wise linear regression on modulation to parametrically increasing WM load revealed significant effects of age in the bilateral inferior parietal lobes such that increasing age was associated with less modulation (for a similar result see Kennedy et al., 2017). Critically, there was also a significant effect of Aβ2 on modulation. The significant negative quadratic effects of Aβ on modulation occurred in two large clusters: bilateral lateral cerebellum (spanning Crus 1 and Crus 2) and bilateral subcortical nuclei including basal ganglia/midbrain/thalamus (spanning portions of caudate, putamen, globus pallidus, nucleus accumbens, thalamic medial dorsal and ventral medial nuclei, and midbrain substantia nigra and ventral tegmental area) (see Table 2 and Figure 1). This quadratic relationship indicated that within these brain regions, individuals with slightly increased Aβ exhibited increased modulation of activation to difficulty, compared to individuals with lower Aβ; in individuals with the highest Aβ, however, modulation began to transition to reduced modulation. A significant positive, linear effect of Aβ burden was found in the same regions as the quadratic effect, and after masking out voxels with significant quadratic effects, there were no regions exhibiting only a significant linear effect of Aβ. There were also no significant non-linear effects in the negative direction and no significant negative linear effects.
Table 2.
Cluster Peaks for Quadratic Effect of Amyloid on BOLD Modulation to WM Load
| Cluster Label | k | x | y | z | Cluster FWEp |
|---|---|---|---|---|---|
| L/R Basal Ganglia (caudate, putamen, nucleus accumbens, globus pallidus), L/R Thalamus (R ventral lateral, R medial dorsal), L/R Midbrain (substantia nigra, ventral tegmental) | 544 | 0 | −33 | −3 | .042 |
| −3 | −27 | −18 | |||
| −9 | −18 | −21 | |||
| L Cerebellum (L Crus 1, L Crus 2) | 484 | −54 | −57 | −33 | .049 |
| −45 | −75 | −39 | |||
| −3 | −78 | −18 | |||
| R Cerebellum (R Crus 1, R Crus 2), R Fusiform Gyrus | 287 | 45 | −72 | −33 | .091* |
| 45 | −51 | −24 | |||
| 36 | −78 | −39 | |||
Note: k – cluster extent number of voxels; L – Left; R – Right. Cluster-wise p-values obtained from SnPM using family-wise error (FWE) correction.
Note that all clusters are significant using SPM’s cluster correction, ps < .012, thus we report all clusters for completeness.
Figure 1. Brain regions exhibiting a quadratic effect of Aβ on BOLD Modulation to parametrically increasing working memory load.
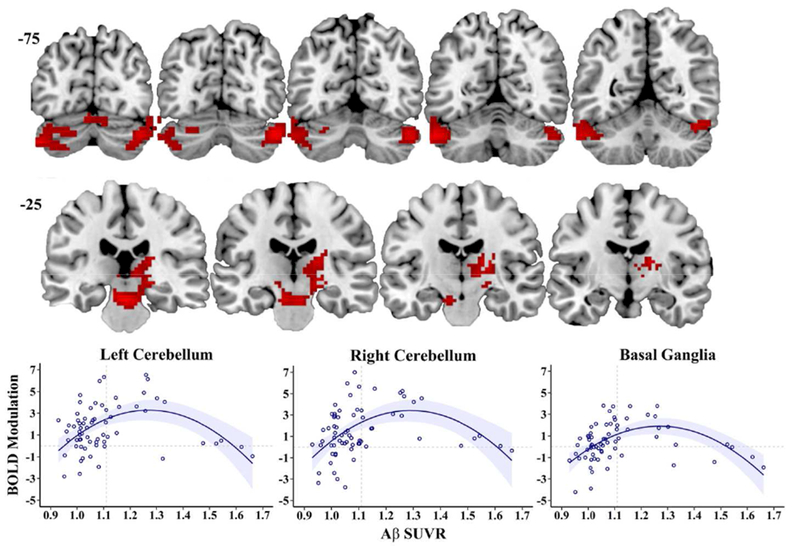
Aβ specific uptake value ratio (SUVR) shows a nonlinear association to BOLD modulation (slope of BOLD change from 0-2-3-4 back conditions) in bilateral cerebellum (top row) and bilateral subcortical nuclei (basal ganglia/thalamus, midbrain; second row). Compared to individuals with lower Aβ, slightly elevated Aβ is associated with greater modulation of activation; Individuals with greater Aβ show lesser modulation of activation (bottom row). The nonlinear BOLD modulation effect remains the same after controlling for gray matter volume and thickness. Quadratic lines shown with 95% confidence interval band. Slice numbers (−75 and −25) continue anteriorly in 5mm increments from left to right images.
Effects of Aβ burden and BOLD Modulation on Cognitive Performance
To examine whether altered modulation was associated with cognitive performance we tested its effects on three markers of performance: task accuracy, EF construct, and WM construct. The analysis predicting in-scanner task accuracy (with age, Aβ, modulation, and their interactions as predictors) indicated a significant negative effect of age on accuracy (t(63) = −2.15, p = .035) and, more importantly a significant positive relationship of modulation on task accuracy (t(63) = 2.86, p = .006). For EF we found a similar pattern of results such that there was a significant negative relationship between age and executive function (t(63) = −3.09, p = .003), and also a significant positive relationship between modulation and executive function (t(63) = 2.42, p = .018). In the WM model, we found no significant effects that predicted WM performance (ps > .21). In none of the three models were there significant relationships of Aβ nor an Aβ by modulation interaction (ps > .22). Overall, these results suggest that beyond the effects of age and Aβ, greater modulation to n-back difficulty is associated with better cognitive performance assessed both in and out of the scanner (see Figure 2).
Figure 2. BOLD Modulation Relates to Behavior.
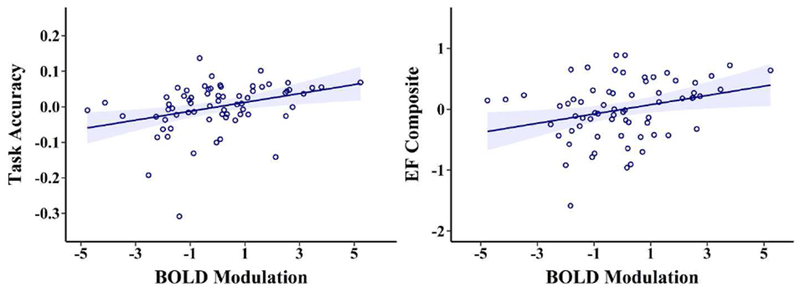
The greater an individual modulates BOLD activation to increased working memory load, the higher the average accuracy during the n-back task (left panel; t(63) = 2.86, p = .006) and the higher the executive function (EF) composite score performance (right panel; t(63) = 2.42, p = .018). All data are plotted as residuals, adjusted for model variables.
Overall Effects of Task on BOLD Response
To place the quadratic Aβ effect in context, we tested the intercept of the fMRI model (i.e., the estimated positive and negative slope across n-back difficulty at the mean of all other variables). Several regions increased in modulation (both in positive and negative modulation) in response to increasing WM load (for similar results see also Kennedy et al., 2017). Regions of increased positive modulation in response to increased WM load included the bilateral cerebellum, bilateral basal ganglia/thalamus, bilateral parietal cortex, and bilateral frontal cortex (see Figure 3). Regions that increased in negative modulation included a set of regions typically associated with the default mode network: the bilateral middle temporal gyrus, bilateral medial frontal, and bilateral cingulate (see Figure 3).
Figure 3. Increases in Modulation as a function of N-Back Load.
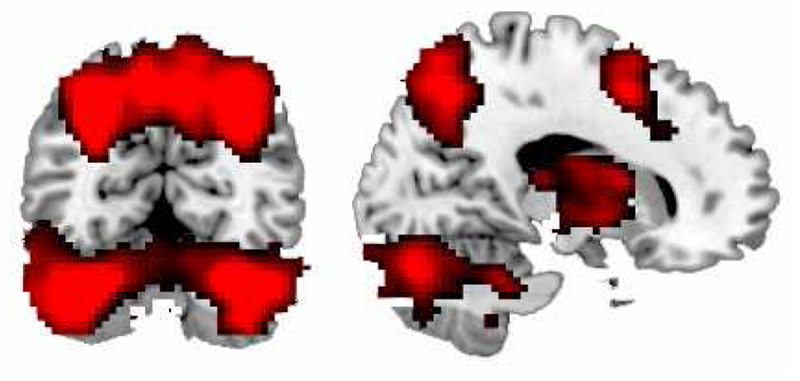
Illustrated is the effect of activation in response to increasing n-back load for older adults at mean age and mean Aβ derived from the full model with age, Aβ, and the quadratic effect of Aβ. Regions of increased modulation in response to increased working memory load include the bilateral cerebellum, bilateral basal ganglia/thalamus, bilateral parietal cortex, and bilateral frontal cortex.
Effect of Covariates on Model Results
To test for effects of brain structure differences on the findings, we tested identical models after controlling for either gray matter volume or gray matter thickness. The model results were unaffected by including grey matter volume or thickness as covariates. Prior research has established that APOEε4 is associated with greater Aβ burden in healthy individuals (for review see Liu, Kanekiyo, Xu, & Bu, 2013) and that APOEε4 is associated with modulation (Foster, Kennedy, & Rodrigue, 2017). Therefore, to assess the potential impact of APOE status on the model results between Aβ and modulation, we again tested the same model including APOE status as a dichotomous variable in the smaller sample of individuals with APOE genotyping, Amyloid PET imaging, and fMRI data (n = 64). We found the same nonlinear association after controlling for APOE status in the same two clusters, however, in this model, the subcortical nuclei cluster was reduced to marginal significance (p = .064, k = 456) and the cerebellar clusters became non-significant (right cerebellum p = .196, k = 147; left cerebellum p = .398, k = 61). However, the peak voxel activations in the cerebellum remained similar, suggesting the overall cluster size dropped with the reduction of 4 participants and the additional covariate. This is somewhat expected given that APOE status is a proxy of Aβ and the shared variance between the two measures would decrease significance of the model.
Discussion
In the present study we tested the hypothesis that the relationship between beta-amyloid deposition and dynamic range of BOLD modulation to parametrically increasing working memory load would be nonlinear in nature, explaining the phenomenon of previous studies reporting elevated Aβ as associated with either increases or decreases in activation during fMRI task-based studies. We previously reported this relationship in a spatial distance judgment task, where individuals with no Aβ demonstrated increased deactivation to difficult judgments, but as Aβ levels increased, transitioned from decreased-to-increased deactivation to difficult judgments, suggesting a nonlinear (quadratic) dose-response relationship between BOLD activity and Aβ (Foster et al., 2018). Similarly, Schultz et al. (2017) found an interaction between Aβ and task-free fMRI that suggested a nonlinear association. Here we sought to replicate this nonlinear association in the same sample of individuals in an n-back task, and to extend our previous finding to dynamic range of BOLD response (i.e., modulation) to increasingly challenging working memory load.
These results fit well with the MCI and AD literature which finds across several studies that in early stages of MCI, there is a hyperactivation to episodic memory tasks in the scanner (e.g., Celone et al., 2006; Dickerson et al., 2005; Foster et al., 2016), but a hypoactivation to the same tasks in individuals with AD (Bosch et al., 2010; Celone et al., 2006; Dickerson et al., 2005; Sperling et al., 2010; for review see Sperling, 2011). This hyper- to hypo-activation was thought, thus, to occur later in the disease pathology, but this recent research suggests that this transition is influenced by amyloid level rather than disease state and can be observed as early as preclinical stages (Foster et al., 2018; Schultz et al., 2017). Early on, this hyperactivation was interpreted as a potentially beneficial, compensatory property of brain function’s response to beta-amyloid accumulation (Dickerson & Sperling, 2008; Sperling et al., 2009). However, individuals demonstrating this hyper-activation showed steeper longitudinal cognitive decline than those who did not show this increased pattern (Dickerson et al., 2004; Miller et al., 2008; O’Brien et al., 2010; Sperling et al., 2010), suggesting that hyperactivation was detrimental in nature (for discussion see Sperling et al., 2011). We recently demonstrated that the transition from hyper-activation to hypo-activation with increasing Aβ, which appears as pseudonormalization, moderates the effect of task deactivation on task accuracy, such that in individuals with clinically elevated Aβ (i.e., SUVR > 1.11) greater deactivation was associated with higher task accuracy, but that at the highest levels of Aβ, greater deactivation was associated with lower task accuracy (Foster et al., 2018). However, we caution that in these cross-sectional designs no temporal order between the effects can be assessed and it is plausible that an alternative potential mechanism of altered brain function as a facilitator of amyloidosis could be true.
The current study extends this amyloid relationship to positively task-activating regions, showing a quadratic function between amyloid level and the dynamic range of modulation to task difficulty. Specifically, we found that in subcortical brain regions at key connections in the pathways of the working memory network (bilateral lateral cerebellum, basal ganglia including portions of caudate, putamen, globus palladus, and nucleus accumbens, midbrain/brainstem including substantia nigra and ventral tegmental area), suprathreshold levels of amyloid were associated with increases in modulation to WM load, whereas as Aβ level increased, modulation to WM load in these key regions decreased. As it is not expected that Aβ is exerting local spatial effects on BOLD activity, it is interesting to consider these subcortical regions as intermediate along the pathways in which these nuclei receive and send projections. The caudate nucleus and dorsomedial nucleus of the thalamus are intermediaries in the dopaminergic pathways that originate in the substantia nigra and the ventral tegmental area and terminate in prefrontal and parietal association cortices. Crus I and II of the cerebellum participate in loops through the thalamus to the prefrontal (and likely) posterior parietal association cortices.
The current findings, then may suggest that amyloid may be also exerting indirect early effects (i.e., preclinical) near the origins of the dopaminergic systems (nigrostriatal from substantia nigra to striatum to neocortical areas, and mesocortical from ventral tegmental area to striatum to neocortical areas) which might affect function at the cortical targets (i.e., frontoparietal). Indeed, in an earlier study utilizing a different sample, we found that aging across the lifespan of the cognitive control system (in a difficult/ambiguous semantic judgment task) followed a highly similar pattern, with early adulthood differences in prefrontal cortices, midlife differences in basal ganglia/nucleus accumbens/dorsomedial nucleus of thalamus, and later life differences in midbrain and brainstem dopaminergic regions of the substantia nigra and the ventral tegmental area (Kennedy et al., 2015). While that study did not take amyloid into account, it suggests that the aging gradient from dopaminergic targets (prefrontal) to more ventral dopamine pathway origin regions is followed in this working memory task, and is influenced by beta-amyloid level.
The effects on the lateral cerebellum are also interesting given the role of the cerebellum in working memory and executive function (Stoodley & Schmahmann, 2008). The lateral cerebellum, especially Crus I/Crus II as we find here, makes connections through the dentate nuclei and then forms fronto-cerebellar loops. While less well understood, this region also forms part of the cortico-limbic-cerebellar circuitry that includes dorsolateral PFC, posterior/inferior parietal, and anterior cingulate cortices, again, regions central to working memory function (and disrupted in schizophrenia; Li et al., 2018).
Interestingly, a few studies have begun to develop compelling links between beta-amyloid and dopamine function. In animals, Aβ provokes an excitation/inhibition imbalance in limbic/cingulate cortex (Ren et al., 2018) and modulates presynaptic neurons’ ability to control dopamine release (Wu, Khan, & Nichols, 2007) suggesting Aβ plays a regulating role in synaptic signaling, perhaps through conversion of long term potentiation to long term depression (Moreno-Castilla et al., 2016). Intriguingly, in APP mouse models of AD, amyloid has a deleterious effect on ventral tegmental (but not substantia nigra) dopamine neuron loss at pre-plaque stages, further suggesting an early role of amyloid in the pathogenesis of AD (Nobili et al., 2017). In humans, this association is almost unexplored, but it appears to not be related to dopamine transporter availability (Rieckmann et al., 2016) in the striatum, however the association may instead be taking place in more ventral dopaminergic pre-synaptic or post-synaptic receptor locations in the midbrain. Though these ideas are speculative given the current data, much research is needed to pursue these intriguing associations among dopaminergic networks and beta-amyloid deposition.
The current study also finds that greater modulation to WM load in these subcortical and cerebellar regions is associated with better cognitive performance both in- (task accuracy) and out- (executive function) of the scanner. This is in line with prior findings (Elman et al., 2014), however, it is not likely that this represents a compensatory response due to Aβ burden. In our prior finding of a non-linear relationship between Aβ and BOLD activity (Foster et al., 2018), we found an interaction between Aβ and de-activation when predicting cognition; however, in the current study we find an effect between modulation and cognitive performance. These two effects represent the same underlying relationship, yet the differences are driven by whether the region being examined activates or deactivates in response to the task. To elaborate, for a region that deactivates, the two extremes of the quadratic relationship (individuals who are deactivating) represent different expectations for cognition, such that deactivation in individuals without Aβ represents the expected neural activity, however, deactivation in individuals with the greatest Aβ represents pathological activity. Therefore, the relationship between amyloid and modulation to cognition flips as a function of these two variables. In contrast, for a region that activates, the two extremes of the quadratic relationship represent similar expectations for cognition. Individuals who have no significant Aβ burden but who are not activating these regions are expected to have poorer cognition (Kennedy et al., 2017), and individuals with significant Aβ burden who are not activating these regions are also expected to have poorer cognition. Put another way, individuals in the high-Aβ group who show the greatest activation also have the least Aβ, thus the positive relationship to behavior could simply represent that these individuals carry less pathology. Thus, these seemingly different relationships to cognition most likely represent the same underlying effect, and further, based on the combination of findings, it is not likely that the positive relationship between modulation and cognition represents a compensatory neural response. It is more probable that the Aβ-driven hyperactivity represents a fundamental property of Aβ’s effect on neural function and that the earliest increases in Aβ drive hyperactivity while further increases in Aβ drive the opposite effect.Indeed, in task-free fMRI, Schultz et al. (2017) suggest that amyloid deposition is associated with increased connectivity, whereas in amyloid-positive individuals tau accumulation is associated with decreased connectivity, suggesting a nonlinear pattern of hyperconnectivity followed by hypoconnectivity in preclinical individuals.
In sum, we replicate previous findings of a nonlinear association between beta-amyloid and BOLD response in cognitively normal middle-aged and older adults. Importantly, the current findings extend our previous results in several ways: from task negative regions to task positive regions, from response to difficult judgments to dynamic range of modulation to parametric difficulty, and from a non-verbal task (spatial distance judgments) to a verbal (n-back working memory load) cognitive task, while holding individual participant differences constant by replicating this effect in the same sample. Taken together, we believe these nonlinear findings help reconcile seemingly discrepant effects of amyloid on brain function (i.e., increases and decreases) and this nonlinear increase-to-decrease of activity reflects a fundamental property of the brain’s response to various levels of beta-amyloid pathology that is task-independent in its manifestation and is detrimental to cognitive function.
Acknowledgements
Supported in part by grants from the National Institutes of Health (AG-036848, AG-036818, AG-056535), the Alzheimer’s Association (NIRG-397220) and by an Investigator Initiated Trial grant from Eli Lilly and Company for the Amyvid ligand. We thank Marci M. Horn and David A. Hoagey for assistance in PET image processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avants BB, Tustison NJ, Song G, & Gee JC (2009). ANTS: Advanced Open-Source Normalization Tools for Neuroanatomy. Penn Image Computing and Science Laboratory. [Google Scholar]
- Bosch B, Bartres-Faz D, Rami L, Arenaza-urquijo EM, Fernandez-Espejo D, Junque C, … Molinuevo JL (2010). Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex, 46, 451–461. doi: 10.1016/j.cortex.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, … Sperling RA (2006). Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. The Journal of Neuroscience, 26(40), 10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, … Skovronsky DM (2011). Use of Florbetapir-PET for Imaging β-Amyloid Pathology. JAMA, 305(3), 275–283. doi: 10.1001/jama.2010.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer J (2001). Delis Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, … Sperling RA (2004). Medial temporal lobe function and structure in mild cognitive impairment. Annals of Neurology, 56(1), 27–35. doi: 10.1002/ana.20163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, … Sperling RA (2005). Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology, 65(3), 404–411. doi: 10.1212/01.wnl.0000171450.97464.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, & Sperling RA (2008). Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: Insights from functional MRI studies. Neuropsychologia, 46, 1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, … Jack CR (2016). Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s and Dementia, 12(3), 292–323. doi: 10.1016/j.jalz.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman J. a, Oh H, Madison CM, Baker SL, Vogel JW, Marks SM, … Jagust WJ (2014). Neural compensation in older people with brain amyloid-β deposition. Nature Neuroscience, 17(10), 1316–8. doi: 10.1038/nn.3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B (2012). FreeSurfer. NeuroImage, 62(2), 774–781. doi: 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Foster CM, Addis DR, Ford JH, Kaufer DI, Burke JR, Browndyke JN, … Giovanello KS (2016). Prefrontal contributions to relational encoding in amnestic mild cognitive impairment. NeuroImage: Clinical, 11, 158–166. doi: 10.1016/j.nicl.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CM, Kennedy KM, Horn MM, Hoagey DA, & Rodrigue KM (2018). Both hyper- and hypo-activation to cognitive challenge are associated with increased beta-amyloid deposition in healthy aging: A nonlinear effect. NeuroImage, 166. doi: 10.1016/j.neuroimage.2017.10.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CM, Kennedy KM, & Rodrigue KM (2017). Differential aging trajectories of modulation of activation to cognitive challenge in APOE ε4 groups: Reduced modulation predicts poorer cognitive performance. Journal of Neuroscience, 37(29), 6894–6901. doi: 10.1523/JNEUROSCI.3900-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, Mcintosh AR, & Grady CL (2013). The Modulation of BOLD Variability between Cognitive States Varies by Age and Processing Speed. Cerebral Cortex, 23, 684–693. doi: 10.1093/cercor/bhs055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Lindenberger U, Hoge RD, & Gauthier CJ (2017). Age differences in brain signal variability are robust to multiple vascular controls. Scientific Reports, 7(10149). doi: 10.1038/s41598-017-09752-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakun JG, & Johnson NF (2017). Dynamic range of frontoparietal functional modulation is associated with working memory capacity limitations in older adults. Brain and Cognition, 118, 128–136. doi: 10.1016/j.bandc.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton SK, Chelune GJ, Talley JL, Kay GG, & Curtiss G (1993). Wisconsin Card Sorting Test manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Huijbers W, Mormino EC, Wigman SE, Ward AM, Vannini P, McLaren DG, … Sperling RA (2014). Amyloid Deposition Is Linked to Aberrant Entorhinal Activity among Cognitively Normal Older Adults. Journal of Neuroscience, 34(15), 5200–5210. doi: 10.1523/JNEUROSCI.3579-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, … Trojanowski JQ (2013). Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. The Lancet Neurology, 12(2), 207–216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, … Trojanowski JQ (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology, 9(1), 119–128. doi: 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, … Zetterberg H (2015). Prevalence of Cerebral Amyloid Pathology in Persons Without Dementia: A Meta-analysis. JAMA, 313(19), 1924–1938. doi: 10.1001/jama.2015.4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Boylan MA, Rieck JR, Foster CM, & Rodrigue KM (2017). Dynamic range in BOLD modulation: lifespan aging trajectories and association with performance. Neurobiology of Aging, 60. doi: 10.1016/j.neurobiolaging.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Bischof GN, Hebrank AC, Reuter-Lorenz PA, & Park DC (2015). Age trajectories of functional activation under conditions of low and high processing demands: An adult lifespan fMRI study of the aging brain. NeuroImage, 104, 21–34. doi: 10.1016/j.neuroimage.2014.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Devous MD, Hebrank AC, Bischof GN, & Park DC (2012). Effects of beta-amyloid accumulation on neural function during encoding across the adult lifespan. NeuroImage, 62(1), 1–8. doi: 10.1016/j.neuroimage.2012.03.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Landau SM, Bell RK, & Jagust WJ (2017). Hippocampal activation is associated with longitudinal amyloid accumulation and cognitive decline. ELife, 6(Mci), 1–15. doi: 10.7554/eLife.22978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Liu F, Su Q, Zhang Z, Zhao J, Wang Y, … Guo W (2018). Bidirectional Causal Connectivity in the Cortico-Limbic-Cerebellar Circuit Related to Structural Alterations in First-Episode, Drug-Naive Somatization Disorder. Frontiers in Psychiatry, 9, 162. doi: 10.3389/fpsyt.2018.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-C, Kanekiyo T, Xu H, & Bu G (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nature Reviews Neurology, 9(2), 106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika PK, Hoeft F, Glover GH, & Reiss AL (2009). Methods and Software for fMRI Analysis for Clinical Subjects. Human Brain Mapping. [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, … Sperling R. a. (2008). Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences of the United States of America, 105, 2181–2186. doi: 10.1073/pnas.0706818105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Castilla P, Rodriguez-Duran LF, Guzman-Ramos K, Barcenas-Femat A, Escobar ML, & Bermudez-Rattoni F (2016). Dopaminergic neurotransmission dysfunction induced by amyloid-β transforms cortical long-term potentiation into long-term depression and produces memory impairment. doi: 10.1016/j.neurobiolaging.2016.02.021 [DOI] [PubMed]
- Mormino EC, Brandel MG, Madison CM, Marks S, Baker SL, & Jagust WJ (2012). Aβ Deposition in aging is associated with increases in brain activation during successful memory encoding. Cerebral Cortex, 22(8), 1813–1823. doi: 10.1093/cercor/bhr255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili A, Latagliata EC, Viscomi MT, Cavallucci V, Cutuli D, Giacovazzo G, … D’amelio M (2017). Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nature Communications, 8(14727). doi: 10.1038/ncomms14727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JL, O’Keefe KM, Laviolette PS, Deluca AN, Blacker D, Dickerson BC, & Sperling RA (2010). Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology, 74(24), 1969–1976. doi: 10.1212/WNL.0b013e3181e3966e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Steffener J, Razlighi QR, Habeck C, & Stern Y (2016). β-Amyloid Deposition Is Associated with Decreased Right Prefrontal Activation during Task Switching among Cognitively Normal Elderly. The Journal of Neuroscience, 36(6), 1962–70. doi: 10.1523/JNEUROSCI.3266-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Steffener J, Razlighi R, Habeck C, Liu D, Gazes Y, … Stern Y. (2015). Aβ-related hyperactivation in fronto-parietal control regions in cognitively normal elderly. Neurobiology of Aging, 36(12), 3247–3254. doi: 10.1016/j.neurobiolaging.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, & Mucke L (2010). Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nature Neuroscience, 13(7), 812–818. doi: 10.1038/nn.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW (2007). PsychoPy-Psychophysics software in Python. Journal of Neuroscience Methods, 162(1–2), 8–13. doi: 10.1016/j.jneumeth.2006.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW (2009). Generating stimuli for neuroscience using PsychoPy. Frontiers in Neuroinformatics, 2, 1–8. doi: 10.3389/neuro.11.010.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, & Reuter-Lorenz PA (2007). Age Differences in Deactivation: A Link to Cognitive Control? Journal of Cognitive Neuroscience, 19(6), 1021–1032. doi: 10.1162/jocn.2007.19.6.1021 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas, 1(3), 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, … Acker JD (2005). Regional Brain Changes in Aging Healthy Adults : General Trends, Individual Differences and Modifiers. Cerebral Cortex, 15(11), 1676–1689. doi: 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Ren S-Q, Yao W, Yan J-Z, Jin C, Yin J-J, Yuan J, … Cheng Z (2018). Amyloid β causes excitation/inhibition imbalance through dopamine receptor 1-dependent disruption of fast-spiking GABAergic input in anterior cingulate cortex. Dysfunction of ACC metabolism and functional connectivity are involved in. Scientific Reports, 8(302), 1–10. doi: 10.1038/s41598-017-18729-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieck JR, Rodrigue KM, Boylan MA, & Kennedy KM (2017). Age-related Reduction of BOLD Modulation to Cognitive Difficulty Predicts Poorer Task Accuracy and Poorer Fluid Reasoning Ability. NeuroImage, 147, 262–271. doi: 10.1016/j.neuroimage.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann A, Hedden T, Younger AP, Sperling RA, Johnson KA, & Buckner RL (2016). Dopamine transporter availability in clinically normal aging is associated with individual differences in white matter integrity. Human Brain Mapping, 37(2), 621–631. doi: 10.1002/hbm.23054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Reick JR, Hebrank AC, Diaz-Arrastia R, … Park DC (2012). β-Amyloid burden in healthy aging: Regional distribution and cognitive consequences. Neurology, 78, 387–395. doi: 10.1212/WNL.0b013e318245d295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, … Villemagne VL (2010). Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging, 31(8), 1275–128 doi: 10.1016/j.neurobiolaging.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL, & Shaw RJ (1991). Effects of adult age on structural and operational capacities in working memory. Psychology and Aging, 6(1), 118–127. doi: 10.1037//0882-7974.6.1.118 [DOI] [PubMed] [Google Scholar]
- Schultz AP, Chhatwal JP, Hedden T, Mormino EC, Hanseeuw BJ, Sepulcre J, … Sperling RA (2017). Phases of hyper and hypo connectivity in the Default Mode and Salience networks track with amyloid and Tau in clinically normal individuals. The Journal of Neuroscience, (617), 3263–16. doi: 10.1523/JNEUROSCI.3263-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA (2011). The potential of functional MRI as a biomarker in early Alzheimer’s disease. Neurobiology of Aging, 32(Suppl 1), 1–11. doi: 10.1016/j.neurobiolaging.2011.09.009.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, … Phelps CH (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 280–292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, … Johnson KA (2010). Functional alterations in memory networks in early alzheimer’s disease. NeuroMolecular Medicine, 12(1), 27–43. doi: 10.1007/s12017-009-8109-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, … Johnson KA (2009). Amyloid Deposition Is Associated with Impaired Default Network Function in Older Persons without Dementia. Neuron, 63(2), 178–188. doi: 10.1016/j.neuron.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Mormino EC, & Johnson K (2014). The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron, 84(3), 608–622. doi: 10.1016/j.neuron.2014.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, & Schmahmann JD (2008). Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed]
- Wechsler D (2008) Wechsler Adult Intelligence Scale – Fourth Edition (WAIS-IV). San Antonio, TX: Pearson. [Google Scholar]
- Wu J, Khan GM, & Nichols RA (2007). Dopamine release in prefrontal cortex in response to β-amyloid activation of α7* nicotinic receptors. Brain Research, 1182, 82–89 doi: 10.1016/j.brainres.2007.08.079 [DOI] [PMC free article] [PubMed] [Google Scholar]


