Abstract
Activation of the classical nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway is a common molecular event observed in both human and canine diffuse large B-cell lymphoma (DLBCL). Although the oncogenic potential of the alternative NFκB pathway (ANFκBP) has also been recently identified in DLBCL, its precise role in tumor pathogenesis and potential as a treatment target is understudied. We hypothesized that up-regulation of the ANFκBP plays an important role in the proliferation and survival of canine DLBCL cells, and we demonstrate that the ANFκBP is constitutively active in primary canine DLBCL samples and a cell line (CLBL1). We further demonstrate that a small interfering RNA inhibits the activation of the NFκB pathway and induces apoptosis in canine DLBCL cells. In conclusion, the ANFκBP facilitates survival of canine DLBCL cells, and thus, dogs with spontaneous DLBCL can provide a useful large animal model to study therapies targeting the ANFκBP.
Keywords: Diffuse large B-cell lymphoma, alternative NFκB pathway, canine model, targeting therapy, comparative pathology
Introduction
Non-Hodgkin lymphoma (NHL) is a heterogeneous group of common lymphoid malignancies, which includes ~30 distinct subtypes as determined by the World Health Organization.[1] The incidence of NHL almost doubled in the past 30 years to an estimated 70,800 new cases and 18,990 deaths in the United States in 2014.[2] Diffuse large B-cell lymphoma (DLBCL) is the most common type of NHL. Though the morphologic appearance of individual DLBCL cases is relatively uniform from case to case, the individual biology and clinical responses are heterogeneous. Although modern immunotherapies have improved outcomes for patients with DLBCL,[3,4] greater than 50% of DLBCL patients will not survive 5 years.[5] DLBCL is commonly divided, according to the gene expression profiling, into two distinct molecular sub-types; activated B-cell like (ABC) DLBCL and germinal center B-cell like (GCB) DLBCL subtypes. ABC DLBCLs are believed to derive from plasmablastic B cells exiting the germinal center and GCB DLBCLs are believed to derive from centroblasts in the germinal center, representing very different intracellular oncogenic signaling pathways between DLBCL subtypes.[6]
While the molecular mechanisms contributing to DLBCL have been elusive, persistent activation of the nuclear factor of kappa B cells (NFκB) was identified as a common molecular event that contributes to the pathogenesis of human ABC-DLBCL,[7,8] and to a lesser degree GCB-DLBCL.[9,10] There are two main NFκB activation pathways; the classical (canonical) pathway, which is mediated by p50, p65, and c-Rel, and the alternative (non-canonical) pathway, which is mediated by p52 and RelB.[11,12] The classical pathway is activated by a variety of stimuli, such as tumor necrosis factor alpha (TNFα), interleukin-1 beta (IL-1β), IL-6, bacterial lipopolysaccharide (LPS), and it regulates a multitude of cellular pathways, such as inflammation, the immune response, proliferation, and apoptosis. Particularly in DLBCL, the classical NFκB pathway is constitutively active owing to diverse somatic mutations, genomic amplifications and deletions, and chromosomal translocations.[10,13–16] The alternative pathway of NFκB activation is particularly important in mature B-cells and is engaged by a restricted set of cell-surface receptors that belong to the TNF receptor superfamily, including CD40 ligand (CD40L), BAFF receptor, and the lymphotoxin β receptor. More recently, the aberrant activation of the alternative NFκB pathway and its oncogenic potential in B-cell malignancies, including marginal zone lymphoma, chronic lymphocytic leukemia, and DLBCL, has become evident.[9,10,17–19] However, less is known regarding the significance of alternative NFκB pathway activation in these diseases.
Activation of the NFκB pathway has also been described in canine DLBCL.[20,21] Gaurnier-Hausser et al. reported constitutive activation of the classical NFκB pathway in canine DLBCL. In addition, dogs with chemoresistant DLBCL treated with the NFκB essential modulator (NEMO) binding domain (NBD)-peptide, which selectively blocks the classical NFκB pathway, showed some reduction in tumor burden.[20] Notably, the alternative NFκB pathway was not analyzed in this study. More recently, Mudaliar et al. [21] reported intranuclear p52 staining in 17 of 17 naïve canine DLBCL samples analyzed using immunohistochemistry (IHC), suggesting activation of the alternative NFκB pathway. The potential role of the alternative pathway in canine DLBCL is further supported by our work demonstrating that CD40L is necessary to maintain canine primary DLBCL cells.[22] Based on these observations, we hypothesized that up-regulation of the alternative NFκB pathway is important for proliferation and survival of canine DLBCL cells. Through this work, we have provided new insights into the role of the alternative NFκB pathway in the pathogenesis of canine DLBCL and substantiated a comparative approach to the study of NHL using the dog as a translational model for human DLBCL.
Materials and methods
Cells lines and reagents
The canine DLBCL cell line CLBL1 was obtained from Dr. Barbara Rutgen (University of Vienna, Vienna, Austria) and the human DLBCL cell line OCI-Ly3 was obtained from UHN/Ontario Cancer Institute (Toronto, ON). Raji cells were obtained from ATCC (Manassas, VA). All cells were cultured as previously described.[22] Bay11–7082 and IKK2 inhibitor IV (TPCA-1) were purchased from EMD Chemicals, Inc. (San Diego, CA) and MG-132 was purchased from Sigma Aldrich (St. Louis, MO). NBD-peptide (DRQIKIWFQNRRMKWKKTALDWSWLQTE) and control peptide (DRQIKIWFQNRRMKWKK) were purchased from Imgenex (San Diego, CA).
Primary samples
Primary DLBCL samples were obtained from pet dogs with owner consent with Institutional Animal Care and Use Committee (IACUC) approval (protocols 0802A27363 and 1101A94713) approved by the University of Minnesota IACUC. Primary unstaged DLBCL samples included DLYM-1004, −1107, −1201, −1208, −1209, and −1302, in addition to one stage III DLBCL sample DLYM-1005.
Cell proliferation assay
Cell proliferation/viability was determined using the CellTiter 96® AQueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI) according to the manufacturer’s instructions. Briefly, 5 × 104 CLBL1 cells were cultured in 100 μL of complete medium in 96-well plates. After 72 h, 20 μL of assay solution was added to each well and cells were incubated for another 3–4 h before measuring absorbance at 490 nm using a Wallac Victor2 1420 Multilabel Counter (Perkin Elmer, Waltham, MA).
Luciferase reporter assay
NFκB pathway reporter cells (CLBL1-NFκB-luc cells) were prepared by transfecting CLBL1 with an NFκB reporter gene cassette containing the NFκB consensus sequence followed by firefly luciferase gene (pGL4.32[luc2P/NF-kB-RE/Hygro], Promega) using the 4D-Nucleofector system (Lonza, Allendale, NJ). Cells were initially selected by 400 μg/mL Hygromycin B (Invivogen) for 2 months, and then maintained in 200 μg/mL Hygromycin B. For NFκB inhibitor assays, 1 × 105 CLBL1-NFκB-luc cells were plated in 100 μL of complete medium in 96-well black wall plates, NFκB inhibitors were added, and then 100 ng/mL CD40L was added. After 4 h of incubation, 100 μL of One-Glo solution (Promega) was added to each well and cells were incubated for 10 min at room temperature before measuring chemiluminescence using a Wallac Victor2 1420 Multilabel Counter (Perkin Elmer).
siRNA nucleofection
The siRNA that targets RelB gene (XM_541569.3) was designed and purchased from Sigma-Aldrich; 5′-CUGUGACCGUGAACGUCUUdTdT (RelB siRNA). MISSION® siRNA Universal Negative Control (UNC) #1 was also purchased from Sigma-Aldrich and used as a negative control siRNA. RelB siRNA and UNC siRNA were used at a final concentration of 1000 nM (=10 pmol siRNA per 1 × 105 cells) for nucleofection of CLBL1 cells using the 4D-Nucleofector system (Lonza).
Annexin V staining
CLBL1 cells were treated by RelB or UNC siRNA and cultured for 24 h. Cells were stained with Annexin V (eBiosciences, San Diego, CA) according to the manufacturer’s instructions and analyzed using a BD LSRII flow cytometer (BD Immunocytometry Systems, San Jose, CA). Results were analyzed using FlowJo software (Tree Star, Ashland, OR).
Immunoblotting
Nuclear lysates of lymphoma cells were prepared using the NE-PER Nuclear Protein Extraction Kit (Thermo Fisher Scientific Inc., Waltham, MA). Protein concentrations of the nuclear lysates were determined using the BioRad Protein Assay kit (BioRad, Hercules, CA). IGROV1 cell lysate, used as a positive control for c-Rel expression, was obtained from Cell Signaling (Beverly, MA). Proteins were separated by sodium dodecyl sul-fate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (BioRad). Antibody staining was performed using the SNAP i.d. system (EMD Millipore Billerica, MA) according to the manufacturer’s instructions. Detection was performed using the Odyssey Infrared Imaging System (LI-COR, Lincoln, NE).
Antibodies used were: rabbit anti-human p65 antibody (Cell Signaling Technology), rabbit anti-human p50/p105 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA), rabbit anti-human p50/p105 antibody (EMD Millipore), rabbit anti-human c-Rel antibody (Santa Cruz Biotechnology Inc.), rabbit anti-human RelB antibody (Cell Signaling Technology), rabbit anti-human p52/p100 antibody (Cell Signaling Technology), rabbit anti-human p52/p100 (Thermo Fisher Scientific Inc.), mouse anti-β-actin antibody (Sigma-Aldrich), and normal rabbit IgG (Cell Signaling Technology). Secondary donkey anti-rabbit antibody conjugated to IRDye800 and anti-mouse antibody conjugated to IRDye680 for immunoblotting were purchased from LI-COR.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts of lymphoma cells were prepared as described above. DNA-binding activity of NFκB was analyzed by EMSA using a 5 μg protein extract and a palindromic NFκB binding sequence probe (LI-COR). EMSA supershift assay was done by using 1–2 μg of the same antibodies described for the immunoblotting studies above. Detection was performed using the Odyssey Infrared Imaging System (LI-COR).
Tissue microarray analysis (TMA) of canine DLBCL
The TMA block was built by the University of Minnesota BioNet core using formalin fixed-paraffin embedded (FFPE) specimens from 27 cases of histologically-confirmed canine DLBCL. The primary lymphomas collected from dog ‘patients’ from practices across the United States before treatment with chemotherapy. After cutting sections from the TMA, adequately cellular slides for 23 dogs for p50, 21 dogs for p65, 20 dogs for RelB, and 21 dogs for p52, respectively were available for IHC. Each TMA IHC included 2 canine normal lymph node samples. Two additional canine DLBCL samples were also immunostained for p50, p65, RelB, and p52.
IHC was performed using standard methods. Subsequent steps were automated using an immunohistochemical staining platform (Nemesis, Biocare, Concord, CA). Endogenous peroxidase activity was quenched by slide immersion in 3% hydrogen peroxide solution (Peroxidazed, Biocare) for 10 min followed by a Tris-Buffered Saline Tween 20 (TBST) rinse. A serum-free blocking solution (Background Punisher, Biocare) was placed on sections for 20 min. Blocking solution was removed, and slides were incubated in primary antibody diluted in 10% blocking solution/90% TBST. Rabbit anti-human anti-p65 (Cell Signaling Technology, 1:500), rabbit anti-human p50/p105 (EMD Millipore, 1:6000), rabbit anti-human RelB (Cell Signaling Technology, 1:200) and rabbit anti-human p52/p100 (Thermo Fisher Scientific Inc., 1:150) were incubated for 60 min at room temperature followed by TBST rinse and detection with Novocastra Novolink Polymer Kit (Leica Microsystems Inc., Buffalo Grove, IL) using the manufacturer’s specifications. All slides then underwent TBST rinse and staining with diaminobenzidine (DAB) (Covance, Dedham, MA). Slides were incubated with DAB for 5 min followed by Tris-Buffered Saline (TBS) rinse, then counterstained with CAT Hematoxylin (Biocare, Concord, CA) for 5 min. Slides were then dehydrated and cover slipped.
For digital image analysis, TMA whole slide images were obtained at 40× magnification (0.0625 μm2/pixel) with a ScanScope CS (Aperio ePathology, Leica Biosystems Imaging, Vista, CA). Nuclear translocation and immunohistochemical staining (an indicator of the pathway activation) of p50, p65, RelB, and p52 were summarized as the average staining intensity within nuclei of tumor epithelium multiplied by the percentage of positive nuclei in tumor epithelium (denoted as AvgNuclearOD*%PosNuclei), which has been previously described.[23–25]
Results
Activation of NFκB pathways in canine DLBCL
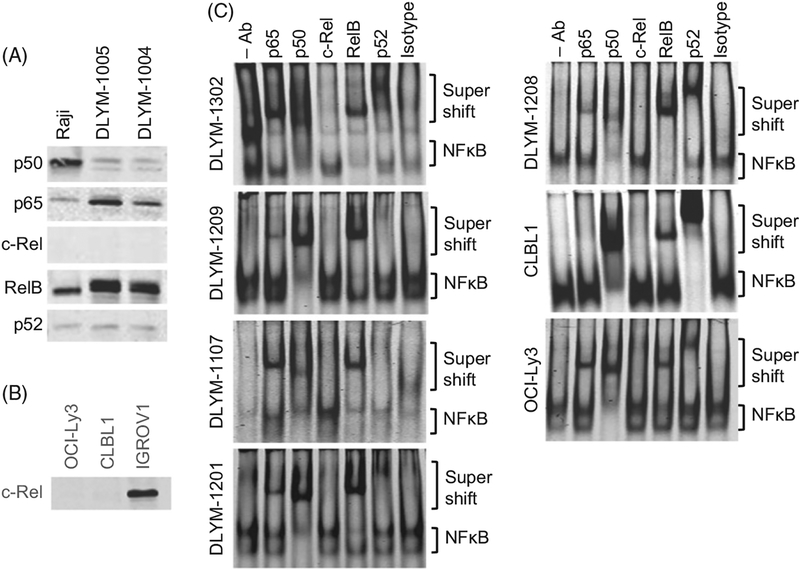
We first analyzed the expression of NFκB proteins (p65, p50, c-Rel, RelB, and p52) in nuclear lysates prepared from two independent canine primary DLBCL cells (DLYM-1004 and DLYM-1005). Human Raji Burkitt lymphoma cells were used as a positive control. We confirmed expression and nuclear translocation of p65, p50, RelB, and p52 in two independent canine primary DLBCL samples as well as in Raji cells; however, c-Rel was not detected in either canine DLBCL cells or Raji cells (Figure 1(A)). To confirm the performance and reactivity of the anti-c-Rel antibody and the detection system, we analyzed expression of c-Rel in human IGROV ovarian cancer cells side-by-side with the canine DLBCL cell line, CLBL1, and the human DLBCL cell line, OCI-Ly3. This experiment confirmed the absence of c-Rel in lymphoma cells, and showed it was detectable in IGROV nuclear lysates (Figure 1(B)).
Figure 1.
Constitutive activation of the NFκB pathway in canine DLBCL. (A) Expression and nuclear translocation of NFκB proteins were analyzed using nuclear lysates prepared from two canine primary DLBCL cells and human Burkitt lymphoma cell line Raji by immunoblotting. (B) Immunoblotting for c-Rel using CLBL1, OCI-Ly3, and IGROV cell lysates. (C) Activation of NFκB pathway (binding of NFκB proteins to the NFκB consensus sequence probe) was analyzed using nuclear lysates prepared from 5 primary canine DLBCL cells, CLBL1 cells, and OCI-Ly3 cells by the EMSA. Super-shifts by anti-NFκB antibodies indicate that the specific NFκB forms active NFκB complexes.
To further determine the activity of the classical and alternative NFκB pathways in canine DLBCL cells, we analyzed nuclear lysates from five independent primary canine DLBCLs (denoted DLYM-1107, 1201, 1208, 1209, and 1302) and from CLBL1 cells for the binding of NFκB proteins to a DNA probe containing the NFκB consensus sequence. OCI-Ly3 cells, which were previously shown to have active classical and alternative NFκB pathways,[7] were used as a positive control. In all samples, we demonstrated constitutive NFκB activity (Figure 1(C), the ‘- Ab’ lane), with weak activity in primary sample DLYM-1107. To characterize the activation status of the classical and alternative pathways, we performed super-shift assays. All five primary canine DLBCL samples demonstrated a supershift of DNA-binding complexes with the addition of the p65, p50, and RelB antibodies (Figure 1(C), lanes 2, 3 and 5). Additionally, three of the five samples (DLYM-1201, 1208, and 1302) showed a supershift with addition the p52 antibody (Figure 1(C), lane 6). In aggregate, these findings indicate constitutive activation of both the classical and alternative NFκB pathways in these cells. Interestingly, p50 and RelB appeared to be the predominant species in the active NFκB complexes seen in canine DLBCL cells. The CLBL1 cells also showed evidence of activation of both NFkB activation pathways with detectable supershifts in the p50, RelB, and p52 lanes (Figure 1(C), lanes 3, 5, and 6). Consistent with previous reports, the human DLBCL cell line OCI-Ly3 also demonstrated activation of both the classical and alternative pathways.[9] In agreement with the immunoblotting results (Figure 1(A)), c-Rel was not observed in the active NFκB complexes for any of the lymphoma cases.
NFκB inhibition prevents proliferation and induces death of canine DLBCL cells
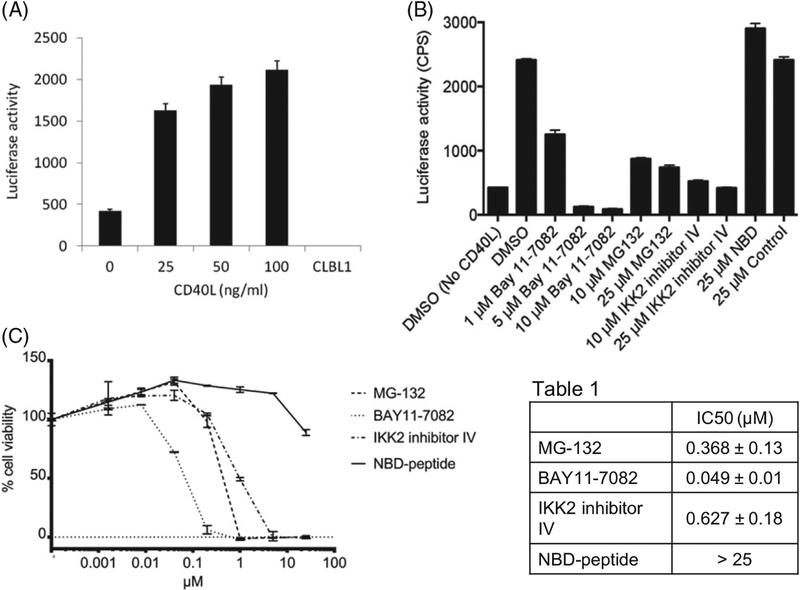
To evaluate NFkB activation in real time, we developed an NFκB reporter cell line by stable transfection of CLBL1 cell with an NFκB reporter gene cassette (NFκB consensus sequence þ luciferase; CLBL1-NFκB-luc). Constitutive NFκB activity was detected in transfected CLBL1 cells (Figure 2(A)) which was enhanced with increasing concentrations of CD40L (Figure 2(A)). No luciferase activity was detected in non-transfected cells. This response was reversed with increasing concentrations of the nonselective (Bay 11–7082 and MG-132) and the classical pathway-selective (IKK2 inhibitor IV) inhibitors. In contrast, the NBD-peptide, which was previously shown to inhibit the classical NFκB pathway in canine DLBCL [20,21] had no effect on CD40L-mediated NFκB activation in CLBL1 cells (Figure 2(B)). To determine the necessity of NFκB activation in canine DLBCL cell survival, we analyzed the cytotoxicity induced by these inhibitors against CLBL1 cells. Consistent with the luciferase findings, the Bay 11–7082, MG-132 and IKK2 inhibitor IV showed strong cytotoxic activity against CLBL1 cells, but the NBD-peptide did not (Figure 2(C)).
Figure 2.
Constitutive activation of the NFκB pathway in canine DLBCL cell line CLBL1. (A) Luciferase activity in CLBL1 NFκB reporter cells cultured for 4 h with or without CD40L. (B) Luciferase activity in CLBL1 NFκB reporter cells cultured for 4 h with 25 ng/ml CD40L in the presence of indicated NFκB pathway inhibitors. (C) Cytotoxicity assay for NFκB pathway inhibitors against CLBL1 cells. Where applicable, error bars represent the 95% confidence interval as determined by either Excel or GraphPad Prism.
Inhibition of alternative NFκB pathway induces apoptosis in canine DLBCL cells
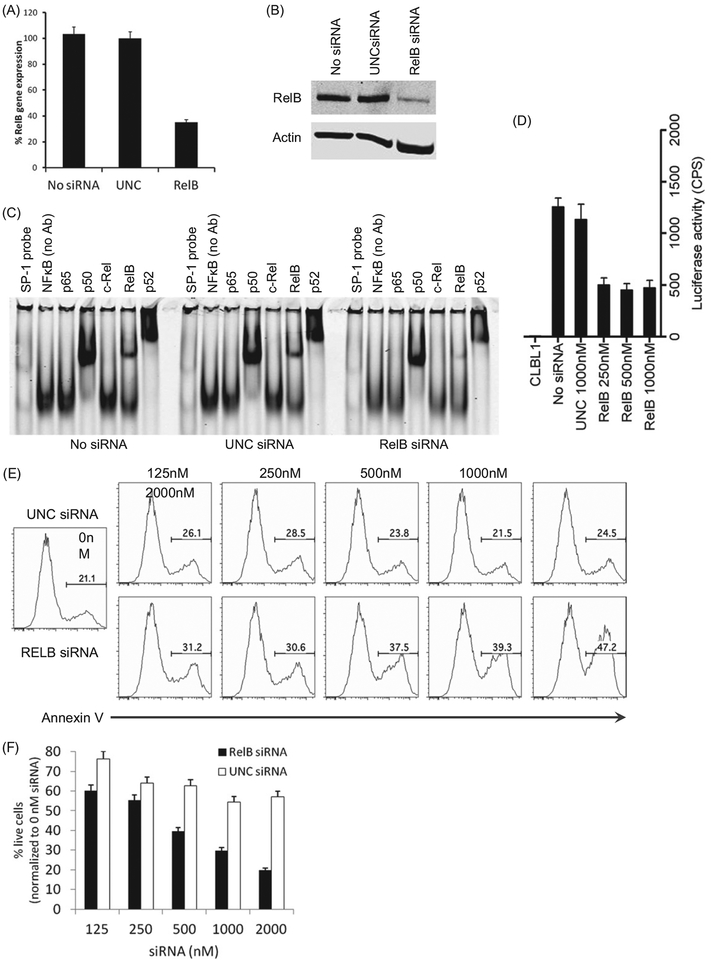
As previous work has shown that knockdown of NIK expression, an upstream regulator of the alternative NFκB pathway, inhibits lymphoma cell growth in human DLBCL cells,[9] we sought to examine the functional consequence of the alternative NFκB pathway inhibition in canine DLBCL. Treatment of CLBL1 cells with RelB siRNA inhibited the expression of RelB mRNA and protein as compared with un-treated cells and cells treated with a universal negative control siRNA (UNC) (Figure 3(A,B)). Similarly, RelB siRNA treatment decreased the amount of bound RelB in NFκB complexes on super-shift assays (Figure 3(C)), but also resulted in decreased p52 binding. Consistent with these biochemical data, inhibition of RelB expression reduced the NFκB activity in CLBL1-NFκB-luc cells (Figure 3(D)) and induced apoptosis of CLBL1 cells in a dose dependent manner (Figure 3(E,F)).
Figure 3.
Inhibition of the alternative NFκB pathway induces apoptosis in CLBL1 cells. (A) CLBL1 cells were transfected with 1000 nM (=10 pmol siRNA per 1 × 105 cells) RelB or UNC siRNA using a nucleofection method. After 24 h, the expression level of RelB gene was analyzed using quantitative RT-PCR. RelB gene expression levels were normalized by beta-actin gene expression among samples. (B) Expression of RelB protein in CLBL1 treated by no siRNA, UNC siRNA, or RelB siRNA was analyzed by immuno-blotting. (C) NFκB pathway in CLBL1-NFκB-luc cells was effectively inhibited by RelB siRNA treatment. (D) Inhibition effect of RelB siRNA was analyzed using nuclear lysates prepared from CLBL1 treated by no siRNA, UNC siRNA, and RelB siRNA using the super-shift EMSA. (E) Induction of apoptosis in CLBL1 cells treated by RelB siRNA was analyzed by Annexin V binding on flow cytometry. (F) CLBL1 cells were treated with UNC or RelB siRNA for 72 h and the cell viability was analyzed using the CellTiter 96® AQueous One Solution Cell Proliferation Assay. Where applicable, error bars represent the 95% confidence interval as determined by either Excel or GraphPad Prism.
Constitutive activation of classical and alternative NFκB pathways in canine DLBCL
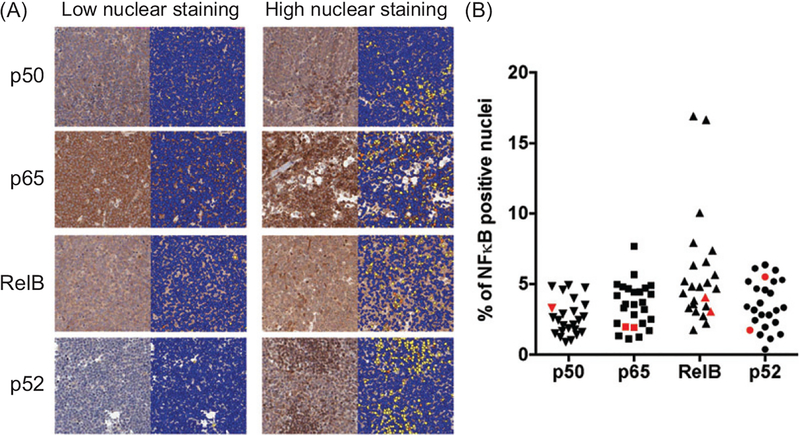
To define the frequency of NFκB activation in primary canine DLBCL, we built a canine DLBCL tissue micro-array containing 27 primary naïve DLBCL samples and examined the nuclear translocation of p65, p50, RelB, and p52 by IHC. Our results show that NFκB proteins were expressed widely in malignant lymph node samples (Figure 4(A)), although nuclear translocation was generally confined to fewer than 10% of the cells (Figure 4(B)). Fifteen of 22 (68%) DLBCL samples showed higher nuclear RelB immunoreactivity than normal controls and 18/22 (82%) had higher p65 expression. In contrast, only 3/23 (13%) and 6/23 (26%) DLBCL samples had increased nuclear translocation of p52 and p50, respectively as compared with control LNs (Figure 4(B)).
Figure 4.
Canine DLBCL TMA. Canine lymphoma TMA slides were stained for p52, p65, and RelB and scanned for the analysis. Manual annotations were drawn to segment the desired TMA spots into individual regions per the TMA maps. Negative annotations were drawn to exclude regions containing poor tissue, staining artifacts, and non-representative tissue (e.g. benign areas). p50, p65, RelB, and p52 antigens were present in tumor areas encompassing nuclear and cytoplasmic staining. The Nuclear aμgorithm was calibrated to the DAB/Hematoxylin stains and nuclear DAB staining was measured in tumor areas. (A) Representative photos with low and high frequency of NFκB nuclear translocation are shown. Blue dots represent unstained nuclei and yellow-orange dots represent positive NFκB staining in nuclei. (B) Summary of the percentage of NFkB positive nuclei in total nuclei among samples. Red color represents the result of normal lymph node samples.
We finally stratified dogs according to the percentage of nuclei with NFκB staining and compared overall survival between ‘NFκB-high’ and ‘NFκB-low’ groups (using median expression level as a cutoff to distinguish high from low categories). The analysis included dogs treated using multi-agents (cyclophosphamide, doxorubicin, vincristine, and prednisolone) chemotherapy protocols (n = 10 for p65 and p52, n = 11 for p50 and RelB) or dogs treated using a single agent doxorubicin protocol (n = 8 for p65, n = 6 for p50, n = 6 for RelB, n = 9 for p52). It should be noted, however, that the primary lymphoma samples used to construct the TMAs were collected from dog ‘patients’ from practices across the United States before treatment with chemotherapy; the group comprising these rare samples did not constitute part of a clinical trial, and the samples and treatments were somewhat heterogeneous. There were no significant differences in survival between the ‘NFκB-high’ and the ‘NFκB-low’ groups (data not shown). There also were no correlations between NFκB activation and age, gender, breed, or histopathological tumor grade, based on mitotic activity (data not shown).
Discussion
Persistent activation of the NFκB pathway has been identified as a common molecular event that contributes to the pathogenesis of human B-cell malignancies. In this study, we demonstrated constitutive activation of alternative and classical NFκB pathways in primary canine DLBCL samples and CLBL1 cells. These findings are consistent with the recent report by Mudaliar et al. [21] that predicted activation of the alternative NFκB pathway in primary canine DLBCL based on gene expression profiling. We further demonstrated that inhibition of RelB using siRNA effectively blocked NFκB activity and induced cell death in CLBL1 cells. The canine DLBCL TMA showed that NFκB proteins were widely expressed in malignant LNs, although nuclear translocation of NFκB components was observed in less than 10% of tumor cells. This most likely reflects the dynamic nature of NFκB protein translocation from the cytoplasm to the nucleus, although we cannot exclude the possibility that this is due to relatively low numbers of cells actively proliferating or to low numbers of cells in the tumors having active NFκB. Despite the fact that no correlation was observed between the percent of cells containing nuclear NFκB and overall survival, NFκB activation is thought to be an important step for lymphomagenesis, and this is consistent with our results showing that knockdown of RelB reduced viability of CLBL1 cells.
NFκB was first identified about 25 years ago as a transcription factor that binds to the enhancer of the kappa light chain gene in B cells. NFκB makes numerous cell-autonomous contributions to the development of mature lymphocytes utilizing two distinct biochemical pathways; the classical NFκB pathway and the alternative NFκB pathway.[26,27] The classical NFκB pathway utilizes p50, p65, and c-Rel, and the alternative NFκB pathway utilizes p52 and RelB for signaling. Constitutive activation of NFkB has been repeatedly shown to be critical to the survival and proliferation of DLBCL cells. Although much of this work has emphasized the classical pathway, the role of the alternative pathway is less understood.
This study provides the first evidence showing constitutive activation of alternative NFκB pathway in canine DLBCL cells, both in culture and primary tumor samples. DLBCL is the most common type of lymphoma in both humans and dogs. This disease has very high morbidity and mortality in both species,[28] so more effective treatments are needed. Persistent activation of the classical NFκB pathway was identified over a decade ago as a common molecular event that contributes to the pathogenesis of human DLBCL.[7,8] More recently, involvement of the alternative NFκB pathway in DLBCL has been repeatedly reported.[9,10,29]
Gaurnier-Hausser et al. previously reported that the classical NFκB pathway was frequently activated in canine DLBCL and that the NBD-peptide, which is a specific inhibitor for the classical NFκB pathway, effectively killed canine DLBCL cells.[15,18] These studies did not analyze the alternative NFκB pathway, so difference between our results and those of Gaurnier-Hausser et al. could be due to heterogeneity in the status and balance of activation of classical and alternative NFκB pathways among canine DLBCL samples. CLBL1 cells did not show activated p65, but had robust activation of the alternative NFκB pathway; thus providing an explanation for the resistance of CLBL1 to NBD-peptide. These findings reiterate the notion that it will be important to analyze the activation status of both the classical and the alternative NFκB pathways for every individual with DLBCL in order to choose the most appropriate targeting strategies.
Based on our EMSA and IHC data, virtually all of the cases in our study demonstrated concurrent activation of the classical and alternative NFkB pathways. While we did not design our experiments to document the incidence of co-activation of both pathways in canine DLBCL, the data are similar to those described for human DLBCL.[10] Pham et al. [9] reported that 14/14 (100%) of human DLBCL cell lines and >80% of human primary DLBCL samples demonstrate the nuclear expression of p65 and p52, indicating co-activation of both pathways. The clinical significance of pathway co-activation needs to examined further, but Zhao et al. [30] suggested that co-expression of both p65 and p52 was associated with significantly worse five-year overall and median survivals when compared to activation of one or neither pathway.
We believe that integrating studies of naturally occurring tumors in dogs into studies of human oncology will remain an effective way to evaluate the novel drug targets and to promote the clinical translation [31,32] However, we must be diligent to understand which mechanisms are evolutionarily conserved and which mechanisms are species specific to efficiently translate knowledge gained by studying canine DLBCL to human DLBCL, and to evaluate the appropriate pharmacological targets and approaches to improve the outcomes of human patients with this disease.
Acknowledgements
This study was supported in part by Morris Animal Foundation grants D10CA-501 (JFM, KLT, MB), D12CA-302 (DI) and D13CA-033 (DI and JFM), a Masonic Cancer Center Hematologic Malignancy Innovations Award (MAL, VB, LJB, JFM), and a grant from the Skippy Frank Fund for Life Sciences and Translational Research (DI and JFM), and grant CHF-1889G from the Golden Retriever Foundation and the AKC Canine Health Foundation (JM, KLT, MB). The Odyssey immunoblotting core was supported through the Comprehensive Cancer Center Support Grant to the Masonic Cancer Center, University of Minnesota (P30 CA077598). JFM is supported by the Alvin and June Perlman Chair in Animal Oncology. The authors gratefully acknowledge donors to the Animal Cancer Care and Research Program of the University of Minnesota that helped support this project. The authors acknowledge Kiersten Jensen for technical help and Mitzi Lewellen for assistance with inventory and database management.
Footnotes
Potential conflict of interest:
Disclosure forms provided by the authors are available with the full text of this article online at http://dx.doi.org/10.1080/10428194.2016.1260122.
References
- [1].Swerdlow SHCE, Harris NL, Jaffe ES, et al. WHO classification of tumours of the haematopoietic and lymph-oid tissues. Lyon: IARC; 2008. [Google Scholar]
- [2].SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) [Internet]. Bethesda, MD: National Cancer Institute; 2012. Available from: http://seer.cancer.gov/csr/1975_2009_pops09/. [Google Scholar]
- [3].Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center bio-markers. J Clin Oncol. 2008;26:2717–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008;26:4587–4594. [DOI] [PubMed] [Google Scholar]
- [5].A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymph-oma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- [6].Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125:22–32. [DOI] [PubMed] [Google Scholar]
- [7].Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. [DOI] [PubMed] [Google Scholar]
- [8].Davis RE, Brown KD, Siebenlist U, et al. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pham LV, Fu L, Tamayo AT, et al. Constitutive BR3 receptor signaling in diffuse, large B-cell lymphomas stabilizes nuclear factor-κB-inducing kinase while activating both canonical and alternative nuclear factor-κB pathways. Blood. 2011;117:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Compagno M, Lim WK, Grunn A, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. [DOI] [PubMed] [Google Scholar]
- [12].Chen L-F, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. [DOI] [PubMed] [Google Scholar]
- [13].Lenz G, Davis RE, Ngo VN, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319:1676–1679. [DOI] [PubMed] [Google Scholar]
- [14].Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lohr JG, Stojanov P, Lawrence MS, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci USA. 2012;109:3879–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hömig-Hölzel C, Hojer C, Rastelli J, et al. Constitutive CD40 signaling in B cells selectively activates the noncanonical NF-kappaB pathway and promotes lymphomagenesis. J Exp Med. 2008;205:1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rossi D, Deaglio S, Dominguez-Sola D, et al. Alteration of BIRC3 and multiple other NF-κB pathway genes in splenic marginal zone lymphoma. Blood. 2011;118: 4930–4934. [DOI] [PubMed] [Google Scholar]
- [19].Rossi D, Fangazio M, Rasi S, et al. Disruption of BIRC3 associates with fludarabine chemorefractoriness in TP53 wild-type chronic lymphocytic leukemia. Blood. 2012;119:2854–2862. [DOI] [PubMed] [Google Scholar]
- [20].Gaurnier-Hausser A, Patel R, Baldwin AS, et al. NEMO-binding domain peptide inhibits constitutive NF-κB activity and reduces tumor burden in a canine model of relapsed, refractory diffuse large B-cell lymphoma. Clin Cancer Res. 2011;17:4661–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mudaliar MA, Haggart RD, Miele G, et al. Comparative gene expression profiling identifies common molecular signatures of NF-kappaB activation in canine and human diffuse large B cell lymphoma (DLBCL). PLoS One. 2013;8:e72591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ito D, Frantz AM, Williams C, et al. CD40 ligand is necessary and sufficient to support primary diffuse large B-cell lymphoma cells in culture: a tool for in vitro preclinical studies with primary B-cell malignancies. Leuk Lymphoma. 2012;53:1390–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rizzardi AE, Johnson AT, Vogel RI, et al. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn Pathol. 2012;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rizzardi AE, Vogel RI, Koopmeiners JS, et al. Elevated hyaluronan and hyaluronan-mediated motility receptor are associated with biochemical failure in patients with intermediate-grade prostate tumors. Cancer. 2014;120:1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rizzardi AE, Rosener NK, Koopmeiners JS, et al. Evaluation of protein biomarkers of prostate cancer aggressiveness. BMC Cancer. 2014;14:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. [DOI] [PubMed] [Google Scholar]
- [27].Gerondakis S, Siebenlist U. Roles of the NF-kappaB pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol. 2010;2:a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ito D, Frantz AM, Modiano JF. Canine lymphoma as a comparative model for human non-Hodgkin lymph-oma: recent progress and applications. Vet Immunol Immunopathol. 2014;159:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Odqvist L, Montes-Moreno S, Sanchez-Pacheco RE, et al. NFkappaB expression is a feature of both activated B-cell-like and germinal center B-cell-like sub-types of diffuse large B-cell lymphoma. Mod Pathol. 2014;27:1331–1337. [DOI] [PubMed] [Google Scholar]
- [30].Zhao Q, Fu W, Jiang H, et al. Clinicopathological implications of nuclear factor kappaB signal pathway activation in diffuse large B-cell lymphoma. Hum Pathol. 2015;46:524–531. [DOI] [PubMed] [Google Scholar]
- [31].Marconato L, Gelain ME, Comazzi S. The dog as a possible animal model for human non-Hodgkin lymph-oma: a review. Hematol Oncol. 2013;31:1–9. [DOI] [PubMed] [Google Scholar]
- [32].Hansen K, Khanna C. Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–880. [DOI] [PubMed] [Google Scholar]