Abstract
Background
Renal cell carcinoma (RCC) is one of the common malignant tumors in the urinary system, which endangers human health for a long time. The past decade, the molecular biology of renal cell carcinoma has made considerable progress, so that we have a more profound understanding of renal cell carcinoma. Molecular biological mechanism of renal cell carcinoma remains to be explored. Evidence indicates that long non-coding RNAs (lncRNAs) may be important players in human cancer progression, including RCC. In this study, we found that a newly discovered pseudogene-derived lncRNA named DUXAP8, a 2107-bp RNA, was remarkably upregulated in RCC.
Material/Methods
Expression of lncRNA DUXAP8 was determined by a qRT-PCR assay in RCC tissues. The proliferation and invasion of RCC cell were measured by a cell proliferation assay and a Transwell invasion assay. Expression of miR-126 was detected by real-time PCR. Interactions between lncRNA DUXAP8 and miR-126 were measured by a luciferase reporter assay and an RNA-pull down assay. In vivo experiments were used to detect tumor formation.
Results
Together, our study not only identifies lncRNA DUXAP8 as a negative regulator of renal cancer with potential clinical value, but also reveals a regulatory mechanism by long non-coding RNAs to control tumor development.
Conclusions
Results from this study provide evidence that lncRNA DUXAP8 enhances renal cell carcinoma progression via downregulating of miR-126, which offers a new approach for the treatment of RCC.
MeSH Keywords: Carcinoma, Renal Cell; MicroRNAs; RNA, Long Noncoding
Background
The development of renal cell carcinoma is a multi-stage and multi-step process including a series of genetic changes including oncogene activation and inactivation of tumor suppressor genes (TSGs) [1]. The loss or inactivation of tumor suppressor gene is one of the important molecular events in tumorigenesis and development [2–4]. Tumors often have chromosomal gene deletions at the tumor suppressor gene locus, showing loss of heterozygosity (LOH) [5]. By detecting and analyzing the tumor LOH and its regulation, tumor suppressor genes can be found in a certain range of chromosome susceptibility genes. In order to understand more about the key molecular events leading to the occurrence and development of renal cell carcinoma, more and more researchers start to explore the mechanism of RCC development.
Recently, numerous studies have revealed that lncRNAs have participate in the development of lots cancers [6–9]. Several reports also found that lncRNAs can regulate gene expression by complementary binding with miRNAs. Lots of studies already highlighted the involvement of lncRNAs in the pathogenesis of diseases including cancers [10–12]. For example, a study demonstrated that miR-335 suppressed RCC cell proliferation and invasion [13]. A newly study also demonstrated that lncRNA FILNC1 was involved in energy metabolism and significantly inhibits renal tumor development [14]. The influence of pseudogene-derived lncRNAs in human cancer may be associated with their ability impacting on cellular functions through various mechanisms. For example, pseudogenes can act as sources of competing endogenous RNAs (ceRNAs) for microRNA sponges, RNA-binding proteins (RBPs), several studies had ascertained that the expression of the pseudogene-derived lncRNA DUXAP8 was upregulated in GC tissues [15], while its function was remained to be unearth on the RCC development and progression.
Material and Methods
Cell culture
Human RCC cell lines (A498, 786-0) were purchased from the Institute of ATCC (Beijing, China). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen, Grand Island, NY, USA)) supplemented with 10% fetal bovine serum (Gibco), 100 U/ml penicillin, and 100 mg/ml streptomycin in humidified air at 37°C with 5% CO2.
RNA extraction and qRT-PCR assay
Add 1 mL of RL lysate to a 3.5 cm diameter culture plate to lyse the cells. Gently use the pipette repeatedly blowing mixed transferred to 1 mL EP tube. RNA extraction was get as the manufacture kit instructions. After the reaction process is completed, the experimental results are exported to an Excel spreadsheet and analyzed using GraphPad Prism 5.0 software.
Cell proliferation assay
Cells were seeded into 96-well plates with a volume of 200 ul per well. In the same culture conditions, cells were cultured 3–5 days (according to the purpose and requirements of the test decided to cultivate time). After incubation for 3–5 days, add 20 ul of MTT solution (5 mg/ml in PBS, pH=7.4) to each well and continue incubation for 4 h, stop the culture and carefully discard the culture supernatant in the wells. For suspension cells, centrifuge and then discard the culture supernatant in the wells. Add 150ul DMSO to each well and shake for 10 min to fully dissolve the crystals.4: Colorimetric: Select 490 nm wavelength, the optical absorption of each hole in the ELISA test instrument, establishing the cell growth curve.
Western blotting
Six-well plate were added 700 μL of protein lysis solution, homogenize with electric homogenizer and let stand on ice. 1) Ultrasonic crushing, 3 s/time, interval 3 s, a total of 3 times. 2) Place in a low temperature, high speed centrifuge (4°C), centrifuge at 12,000 rpm for 10 min. 4) Transfer the supernatant to a new EP tube. Prepared SDS - PAGE plastic, the upper 5% concentrated plastic, the lower 10% separation gel. 3) The imprinted membrane was blocked with 5% nonfat dry milk for 1 h at room temperature, and the corresponding bands were cut according to the size of the target protein. The imprinted membranes were respectively placed in the primary antibody of CED-9 (1: 1000) overnight. Washing the membrane 3 times with 1×TBST solution for 10 minutes. The membrane with the PPARγ protein band was placed in horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (1: 5000) and the membrane with the CED-9 protein band was placed on HRP-labeled rabbit anti-goat IgG (1: 5000) for 1 h at room temperature. 4) Wash the membrane with 1×TBST solution, 10 min/time, 3 times.
Transwell assay
We placed 100–200 μL of cell suspension into the Transwell chamber and added 500 μL FBS or chemokine-containing medium. Cells were routinely cultured for 12–48 h. The medium was discarded, cells were washed 3 times with PBS, and fixed with 4% paraformaldehyde for 10 min. DAP-stained nuclei were counted using fluorescence microscopy to assess cell migration.
MTT assay
Cells transfected with si-NKILA or si-Control were digested by trypsin, collected by centrifuging, and seeded into 96-well plates. Cell suspension concentration was adjusted to 5–10×104/ml. Cells were incubated at 37° C for 16–48 h and we observed the effect of the drug. We added 10 μM of MTT solution (5 mg/ml, 0.5% MTT) to each well and continued incubation for 4 h. After the drug and MTT reacted, we centrifuged the 96-well plates and discarded the culture medium. Then, we carefully performed washing with PBS 2–3 times, then added MTT-containing culture medium. Culturing was stopped, the crystals were dissolved, and the absorbance at 490 nm was measured using a microplate reader (Bio-Rad Laboratories, California, USA).
Luciferase reporter assay
Growth medium was removed from the cells to be assayed. After rinsing the cells twice in PBS buffer, being careful not to dislodge any of the cells, we added a minimal volume of 1×CCLR to cover the cells (250 μl for a 60 mm dish) and incubated them briefly at room temperature. Attached cells were scraped free from the culture dish and the cells and solution were transferred to a microcentrifuge tube for centrifuging for 5 s at 12 000 rpm to pelletize the cell debris. The supernatant (cell extract) was transferred to a new tube and we discarded the pelleted cell debris. We mixed 20 μl of cell extract with 100 μl Luciferase Assay Reagent at room temperature.
Statistical analyses
All experimental results are expressed as mean standard deviation (x±s). Comparisons between the 2 groups were performed using the t test and P<0.05 was defined as statistically significant. GraphPad Prism 5.0 (GraphPad Software, Inc.) software were used for analysis and mapping.
Result
LncRNA DUXAP8 expression is upregulated in RCC tissues
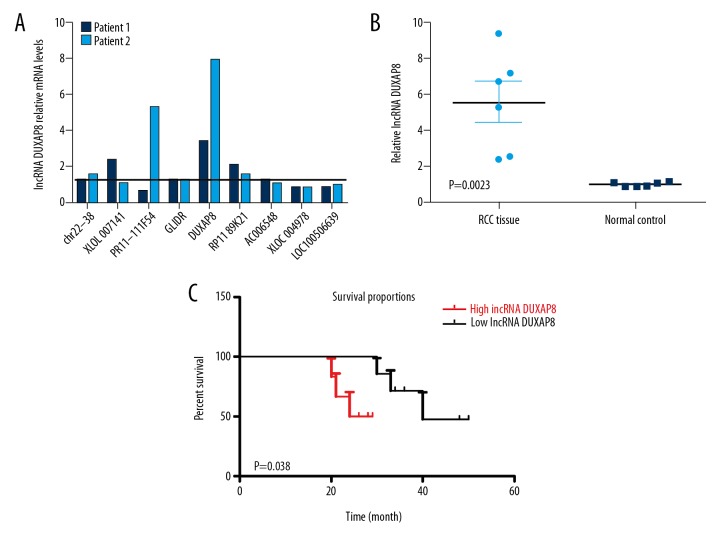
Recently, many studies have indicated that lncRNAs can regulate the development and gene expression by complementary functioning with miRNAs [16–18]. We hypothesized that some lncRNAs can regulate RCC cell progression by binding with miRNAs. Therefore, we used a human lncRNA target prediction tool (DIANA TOOLS) to predict potential lncRNAs that can regulate RCC cell progression and determined the top 14 potential lncRNAs that were under regulation. We found the lncRNA DUXAP8 was changed significantly (Figure 1A). Next, we detected the expression level of lncRNA DUXAP8 in human RCC tissue. The qRT-PCR results showed that lncRNA DUXAP8 was significantly increased in RCC tissue compared with the normal kidney tissues from patients (Figure 1B). Furthermore, our data showed that the RCC patients with high expression level lncRNA DUXAP8 had significantly worse outcomes compared with those in the low lncRNA DUXAP8 group (Figure 1C). Collectively, these data indicate that lncRNA DUXAP8 may have effects on the pathology development of RCC.
Figure 1.
lncRNA DUXAP8 may have effects on the pathology development of RCC. (A) RT-PCR assay was performed to screen 10 potential lncRNAs. lncRNA DUXAP8 was the most appropriate lncRNA to select. Each column is the ratio of cancer tissue/normal tissue. (B) lncRNA DUXAP8 expression in RCC tissues vs. normal renal tissues. (C) Association of lncRNA DUXAP8 expression of 6 RCC patients. P values are given in the figures.
lncRNA DUXAP8 promotes RCC cell proliferation and invasion
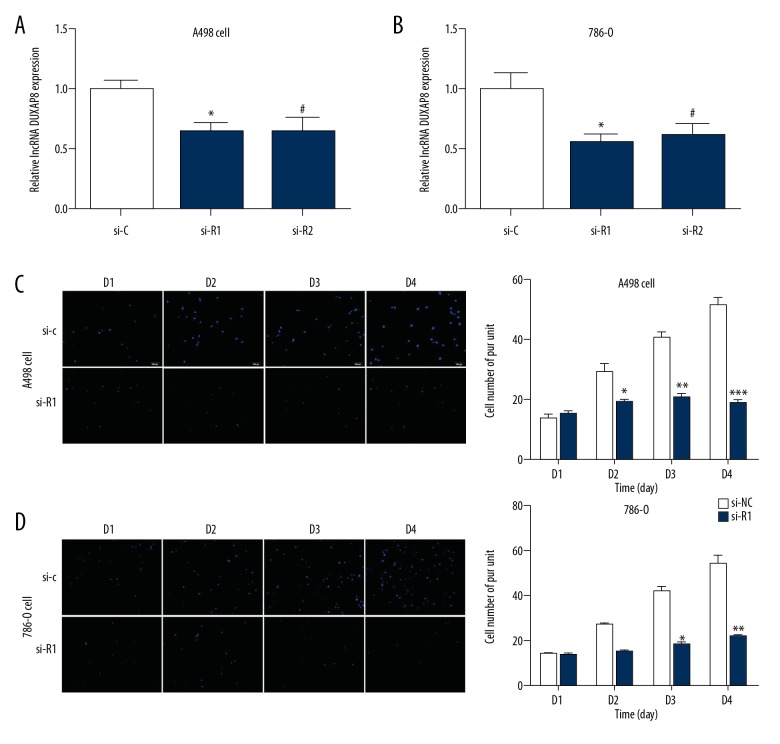
Next, we explore the functional effects of lncRNA DUXAP8 on RCC cells. The RRC cell lines A498 and 786-O cells were transfected with 2 different lncRNAs: DUXAP8-shRNA and (siR-1 and siR-2) and relative control lncRNA DUXAP8-siCtrl (si-C). Next, lncRNA DUXAP8-shRNA and functional lncRNA DUXAP8-siCtrl were transfected into the A498 and 786-O cells for 48 h. The qRT-PCR data revealed that all of the lncRNA DUXAP8 siRNAs effectively entered the cells (Figure 2A, 2B). In addition, the Transwell assays showed that knockdown of lncRNA DUXAP8 expression significantly inhibited the migration of A498 and 786-O cells compared with the scrambled control (Figure 2C, 2D). These results suggest that lncRNA DUXAP8 promotes the invasion of RCC.
Figure 2.
lncRNA DUXAP8 promoted the invasion of lung adenocarcinoma. (A, B) Verification of lncRNA DUXAP8 knockdown by qRT-PCR assay in A498 and 786-O cells. (C, D) Cell growth was measured by a Transwell assay. Each sample was run in triplicate and in multiple experiments for mean ±SEM. * P<0.05, ** P<0.01 compared to controls.
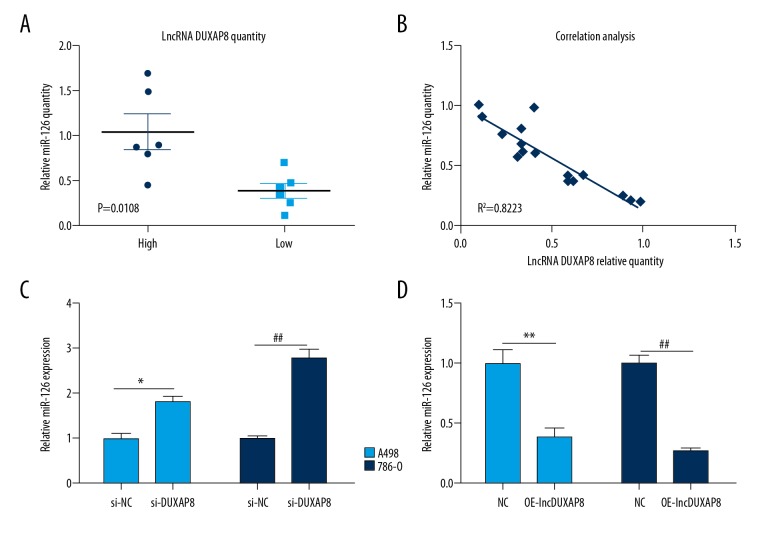
lncRNA DUXAP8 promotes RCC cell proliferation by downregulating miR-126 expression
Many recent studies have found that approximately 20% of lncRNAs can regulate downstream target genes by functioning with 1 or more micro-RNAs [19,20]. To determine the mechanism underlying the lncRNA DUXAP8 expression profile in RCC development, we first assessed the expression of different miRNAs in RCC and paired normal tissues. Interestingly, the expression level of miR-126 was dramatically downregulated in RCC (Figure 3A). A recent study provides important evidence that miR-126 can function as a tumor suppressor in RCC. To investigate whether lncRNA DUXAP8 can regulate miR-126 expression in RCC, the expression of miR-126 and lncRNA DUXAP8 were examined. Q-PCR analysis revealed that lncRNA DUXAP8 expression was negatively correlated with miR-26a level in RCC samples (Figure 3B). To verify whether miR-126 was indeed a target of lncRNA DUXAP8, we first knocked down lncRNA DUXAP8expression in 2 independent RCC cell lines, A498 and 786-O, by siRNA transfection. In both lncRNA DUXAP8 knockdown cell lines, we observed elevated expression levels of miR-126 (Figure 3C), whereas lncRNA DUXAP8 overexpression significantly suppressed the expression of miR-126 in these 2 RCC cell lines (Figure 3D), indicating that miR-126 was downstream of DUXAP8.
Figure 3.
lncRNA DUXAP8 promotes RCC cell proliferation by downregulating miR-126 expression. (A) qRT-PCR assay was performed to detect the expression level of miR-126. (B) The correlation between miR-126 and DUXAP8 level in RCC tumor tissues was analyzed by q-PCR. (C) qRT-PCR assay was performed to detect the expression of miR-126 after knocking down lncRNA DUXAP8in A498 and 786-O cells. (D) qRT-PCR assay was performed to detect the expression of miR-126 after overexpression of lncRNA DUXAP8 in A498 and 786-O cells. Each sample was run in triplicate and in multiple experiments for mean ±SEM. * P<0.05, ** P<0.01 compared to controls.
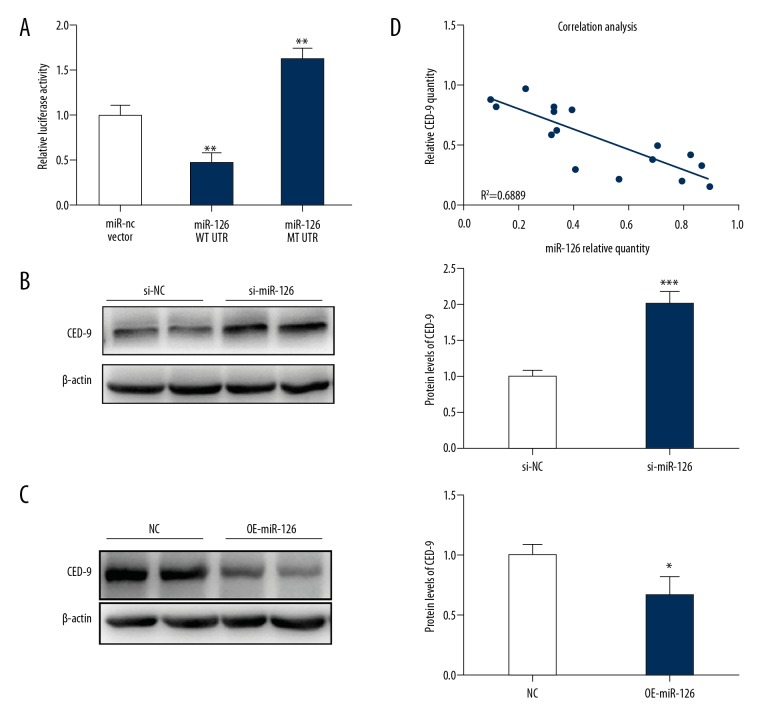
miR-126 targets CED-9 to inhibit RCC tumorigenesis
The above results reveal that lncRNA DUXAP8 inhibited the expression of miR-126 and decreased miR-126 expression in RCC cells. It is very exciting that after identifying the publicly available algorithms (microRNA.org), we found that the CED-9 was one of the potential targets of miR-126a in RCC cells. Thus, we assessed whether lncRNA DUXAP8 influences the process of RCC though regulating CED-9. First, luciferase reporter assays were performed in 293T cells after co-transfecting CED-9 wild type or mutant UTR with miR-126. This data revealed that CED-9 was the direct target of miR-126 (Figure 4A). Western blot assay revealed that knocking down miR-126 increased the expression of CED-9 compared to the control in 786-O cells (Figure 4B), whereas overexpression of miR-126 decreased CED-9 expression (Figure 4C). The Q-RT-PCR analysis also showed that CED-9 expression level was negatively correlated with miR-126 expression level in RCC samples (Figure 4D).
Figure 4.
miR-126 targets CED-9 to inhibit RCC tumorigenesis. (A) Luciferase reporter assays were performed in 293T cells after co-transfecting CED-9 wild-type or mutant UTR with miR-126. (B) The protein level of CED-9 after knocking down miR-126 in 786-O cells. (C) The protein level of CED-9 after over expression of miR-126 in 786-O cells. (D) The correlation between miR-126 andCED-9 level in RCC tumor tissues were analyzed by q-PCR. Each sample was run in triplicate and in multiple experiments for mean ±SEM. * P<0.05, ** P<0.01 compared to controls.
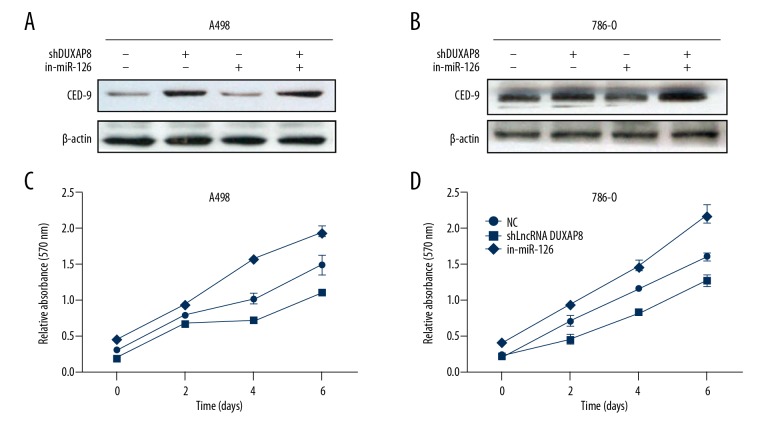
lncRNA DUXAP8 functions by altering miR-126/CED-9 signals to promote RCC cell proliferation and invasion
Previous studies demonstrated that miR-126 inhibited RCC cell progression through the direct suppression of CED-9 [21,22]. Therefore, we examined whether lncRNA DUXAP8 could affect RCC cell proliferation and invasion by altering miR-126/CED-9 signals. Western blot assay revealed that knocking down lncRNA DUXAP8 decreased the expression of CED-9 in A498 and 786-O cells, and this si-lncRNA DUXAP8-suppressed CED-9 expression was partially reversed by adding a miR-126 inhibitor (Figure 5A, 5B). On the other hand, miR-126 inhibitor partially reversed RCC cell proliferation induced by si-lncRNA DUXAP8 in 786-O cells (Figure 5C). As expected, we obtained similar results in A498 cells (Figure 5D) using an MTT assay invasion assay. Together, the results from Figure 4A–4D illustrated that lncRNA DUXAP8 promotes RCC cell proliferation and invasion by altering miR-126/CED-9 signals.
Figure 5.
lncRNA DUXAP8 functions by altering miR-126/CED-9 signals to promote RCC cell proliferation and invasion. (A, B) Protein level of CED-9 was determined after cotransfection with lncRNA DUXAP8-shRNA and miR-126 inhibitor in A498 and 786-O. (C, D) Adding miR-126 inhibitor partially rescued the growth of A498 and 786-O cells transfected with lncRNA DUXAP8-shRNA by a cell proliferation assay. Each sample was run in triplicate and in multiple experiments for mean ±SEM. * P<0.05, ** P<0.01 compared to controls.
Discussion
Renal cancer accounts for about 2–3% of adult malignant tumors, accounting for 80–90% of adult renal malignancies [23]. In general, the incidence rate in developed countries is higher than that in developing countries. Many lncRNAs play vital roles in cancer pathogenesis [24–27]. However, the role and mechanism of lncRNA DUXAP8 [28] in RCC remains unclear.
In the present study, we found that lncRNA DUXAP8 expression was obviously increased in RCC tumor tissues compared with their normal kidney tissues. We also identified that lncRNA DUXAP8 expression was also increased in metastatic RCCs. As expected, lncRNA DUXAP8 knockdown significantly suppressed the A498 and 789-O cell growth. Furthermore, the Transwell assay revealed that lncRNA DUXAP8 knockdown repressed the RCC tumor growth in vivo. These results suggest that lncRNA DUXAP8 acts as an oncogene in RCC tumorigenesis and development.
Numerous studies have shown that lncRNAs acts as a ceRNA to sponge microRNAs to modulate the expression level of microRNA targets in many biological processes. Recently, a new mechanism was discovered in which some lncRNAs and mRNAs interact with each other. In this study, we found that the expression level of microRNA-126 was negatively correlated with the expression level of lncRNA DUXAP8 in RCC tissue samples. MicroRNA-126 has been reported to regulate several types of tumor progression. miR-126 performs converse roles in proliferation and metastasis of different gastric cancer cells. For example, 126miRNA-126 inhibited the growth of lung cancer cells. This prompted us to explore whether miR-126 has an effect on RCC tumor progression and whether lncRNA DUXAP8 inhibits miR-126 to modulate RCC development. To address these questions, we first examined the expression of miR-126 in RCC cells after lncRNA DUXAP8 knockdown. Q-PCR analysis showed that DLX6-AS1 knockdown increased the expression level of miR-126 in 2 RCC cells. Furthermore, we hypothesized that miR-126 overexpression significantly suppressed the growth of RCC cells in vitro. As expected, miR-126 overexpression suppressed the growth of RCC cells. These results suggest that miR-126 acts as a tumor suppressor in RCC tumors. Bioinformatics analysis revealed that CED-9 is the potent predicted target of miR-126. We discovered that miR-126 performs converse roles in proliferation and metastasis of different gastric cancer cells via regulating CED-9 expression. Some studies have found that miRNA-126 inhibits cell cycle progression from G1/G0 to S and that miRNA-126 is frequently lost in colon cancers.
In this study, luciferase reporter assays and Western blot analysis showed that miR-126 directly targets CED-9 and suppresses the protein level of CED-9 in RCC cells. To determine whether CED-9 can restore RCC cell growth, we next co-transfected RCC cells with miR-126 and CED-9. MTT assays revealed that CED-9 can rescue repression of the RCC cell growth induced by miR-126 overexpression.
Conclusions
In conclusion, our study revealed that lncRNA DUXAP8 is an oncogene in RCC. Elevated level of lncRNA DUXAP8 is positively correlated with tumor progression and development. lncRNA DUXAP8 functions as a ceRNA to sponge miR-126 to facilitate RCC progression, partly via the miR-126/CED-9 axis [29]. Thus, our study provides further insight into the molecular mechanism of lncRNAs in RCC tumorigenesis, which can help better understand the pathogenesis and progression of RCC.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Meyer AR, Allaf ME, Rowe SP, Gorin MA. The role of molecular imaging in the characterization of renal masses. Curr Opin Urol. 2018;28(2):159–65. doi: 10.1097/MOU.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 2.Sun M, De Velasco G, Brastianos PK, et al. The development of brain metastases in patients with renal cell carcinoma: Epidemiologic trends, survival, and clinical risk factors using a population-based cohort. Eur Urol Focus. :2018. doi: 10.1016/j.euf.2017.12.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Storkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80:987–89. doi: 10.1002/(sici)1097-0142(19970901)80:5<987::aid-cncr24>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Mouallem NE, Smith SC, Paul AK. Sarcomatoid renal cell carcinoma: Biology and treatment advances. Urol Oncol. 2018;36:265–71. doi: 10.1016/j.urolonc.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Macoska JA, Trybus TM, Benson PD, et al. Evidence for three tumor suppressor gene loci on chromosome 8p in human prostate cancer. Cancer Res. 1995;55:5390–95. [PubMed] [Google Scholar]
- 6.Adams BD, Kasinski AL, Slack FJ. Aberrant regulation and function of microRNAs in cancer. Curr Biol. 2014;24:R762–76. doi: 10.1016/j.cub.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez IG, Nakagawa N, Duffield JS. MicroRNAs as novel therapeutic targets to treat kidney injury and fibrosis. Am J Physiol Renal Physiol. 2016;310:F931–44. doi: 10.1152/ajprenal.00523.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer A, Jung M, Kristiansen G, et al. MicroRNAs and cancer: Current state and future perspectives in urologic oncology. Urol Oncol. 2010;28:4–13. doi: 10.1016/j.urolonc.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Fendler A, Stephan C, Yousef GM, Jung K. MicroRNAs as regulators of signal transduction in urological tumors. Clin Chem. 2011;57:954–68. doi: 10.1373/clinchem.2010.157727. [DOI] [PubMed] [Google Scholar]
- 10.van Solingen C, Seghers L, Bijkerk R, et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med. 2009;13:1577–85. doi: 10.1111/j.1582-4934.2008.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Fan L, Liu S, et al. The promotion of bone regeneration through positive regulation of angiogenic-osteogenic coupling using microRNA-26a. Biomaterials. 2013;34:5048–58. doi: 10.1016/j.biomaterials.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 12.Cui L, Zhou H, Zhao H, et al. MicroRNA-99a induces G1-phase cell cycle arrest and suppresses tumorigenicity in renal cell carcinoma. BMC Cancer. 2012;12:546. doi: 10.1186/1471-2407-12-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang K, Jin W, Song Y, Fei X. LncRNA RP11-436H11.5, functioning as a competitive endogenous RNA, upregulates BCL-W expression by sponging miR-335-5p and promotes proliferation and invasion in renal cell carcinoma. Mol Cancer. 2017;16:166. doi: 10.1186/s12943-017-0735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao ZD, Han L. Energy stress-induced lncRNA FILNC1 represses c-Myc-mediated energy metabolism and inhibits renal tumor development. Nat Commun. 2017;8(1):783. doi: 10.1038/s41467-017-00902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma HW, Xie M, Sun M, et al. The pseudogene derived long noncoding RNA DUXAP8 promotes gastric cancer cell proliferation and migration via epigenetically silencing PLEKHO1 expression. Oncotarget. 2017;8:52211–24. doi: 10.18632/oncotarget.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami Y, Yasuda T, Saigo K, et al. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537–45. doi: 10.1038/sj.onc.1209283. [DOI] [PubMed] [Google Scholar]
- 17.Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–98. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Sampson VB, Rong NH, Han J, et al. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762–70. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- 19.Fan C, Tang Y, Wang J, et al. Role of long non-coding RNAs in glucose metabolism in cancer. Mol Cancer. 2017;16:130. doi: 10.1186/s12943-017-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao ZD, Zhuang L, Gan B. Long non-coding RNAs in cancer metabolism. Bioessays. 2016;38:991–96. doi: 10.1002/bies.201600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lascarez-Lagunas LI, Silva-Garcia CG, Dinkova TD, Navarro RE. LIN-35/Rb causes starvation-induced germ cell apoptosis via CED-9/Bcl2 downregulation in Caenorhabditis elegans. Mol Cell Biol. 2014;34:2499–516. doi: 10.1128/MCB.01532-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert G, Munoz N, Melchiorre M, Sanchez F, Lascano R. Expression of animal anti-apoptotic gene Ced-9 enhances tolerance during Glycine max L.-Bradyrhizobium japonicum interaction under saline stress but reduces nodule formation. PLoS One. 2014;9:e101747. doi: 10.1371/journal.pone.0101747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodi FS, Ballinger M, Lyons B, et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): Refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018;36(9):850–58. doi: 10.1200/JCO.2017.75.1644. [DOI] [PubMed] [Google Scholar]
- 24.Xu M, Gu M, Zhang K, et al. miR-203 inhibition of renal cancer cell proliferation, migration and invasion by targeting of FGF2. Diagn Pathol. 2015;10:24. doi: 10.1186/s13000-015-0255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu GJ, Dong YQ, Zhang QM, et al. miRNA-221 promotes proliferation, migration and invasion by targeting TIMP2 in renal cell carcinoma. Int J Clin Exp Pathol. 2015;8:5224–29. [PMC free article] [PubMed] [Google Scholar]
- 26.Yang FQ, Zhang HM, Chen SJ, et al. MiR-506 is down-regulated in clear cell renal cell carcinoma and inhibits cell growth and metastasis via targeting FLOT1. PLoS One. 2015;10:e0120258. doi: 10.1371/journal.pone.0120258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwok ZH, Tay Y. Long noncoding RNAs: Lincs between human health and disease. Biochem Soc Trans. 2017;45:805–12. doi: 10.1042/BST20160376. [DOI] [PubMed] [Google Scholar]
- 28.Sun M, Nie FQ, Zang C, et al. The pseudogene DUXAP8 promotes non-small-cell lung cancer cell proliferation and invasion by epigenetically silencing EGR1 and RHOB. Mol Ther. 2017;25:739–51. doi: 10.1016/j.ymthe.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–70. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]