Abstract
Efforts to generate tissue-engineered anterior cruciate ligament replacements are limited by a lack of methods to derive mature ligament cells. Viral overexpression of the tendon/ligament marker scleraxis (Scx) can drive cell differentiation; however, the use of viral vectors hampers translation to clinical use. In this study, C3H10T1/2 cells were transiently transfected with expression vectors containing the full-length murine Scx cDNA and cultured in three-dimensional collagen hydrogels under static or cyclic strain for up to 14 days. β-galactosidase (LacZ) transfected cells served as controls. Cell morphology and gene expression for ligament-related genes, in addition to contraction (hydrogel width), mechanical properties, and glycosaminoglycan (GAG) and DNA content of hydrogels, were quantified and compared over time, between Scx and LacZ groups, and between static and cyclically strained constructs. Increased Scx expression was maintained for the entire 14-day study in both static and cyclically strained constructs. In static culture, overexpression of Scx resulted in greater cell elongation and construct contraction compared to LacZ controls. There were no differences in gene expression, DNA, or GAG content between Scx and LacZ constructs cultured under static conditions and no differences in DNA content between Scx and LacZ constructs. When exposed to cyclic strain, Scx-overexpressing cells maintained the elongated phenotype exhibited in static constructs, increased GAG production compared to static culture, and increased expression of the ligament-related genes collagen type I, decorin, and tenascin-C compared to strained LacZ controls. Cyclically strained constructs containing Scx-overexpressing cells had increased maximum load and stiffness compared to LacZ controls. The maintenance of increased Scx expression throughout the 14 day study and subsequent increases in ligament marker gene expression and mechanical properties with cyclic, but not static strain, suggest that transient transfection may be a viable alternative to viral transduction of Scx for ligament engineering studies and support a synergistic effect of Scx and mechanical strain on driving early ligament cell differentiation.
Keywords: : ligament, scleraxis, mechanical strain
Introduction
Anterior cruciate ligament (ACL) injuries are common orthopedic injuries, frequently requiring surgical intervention to reconstruct or replace the damaged tissue.1–3 Current ACL reconstruction techniques include the use of allograft or autograft tissues; however, these procedures are not without complications. Autografts are limited by the availability of suitable donor tissue and frequently lead to chronic pain and morbidity at the donor site.4 Allografts risk delayed integration, immunogenic graft rejection, and disease transmission.5 Tissue engineering offers an alternative that circumvents these problems and potentially provides a tailored, patient-specific replacement tissue.
The success of a tissue-engineered ACL relies on the ability of the cells within the replacement tissue to recapitulate the unique biomechanical properties of native ligament through the creation of a tissue-appropriate extracellular matrix (ECM). The ACL is composed mainly of parallel bundles of collagen type I, which lend tensile strength to the tissue, and also a higher percentage of collagen type III, smaller diameter fibrils, and glycosaminoglycans (GAG) compared to tendon.2,6 These unique features increase the elasticity of the ACL and allow it to undergo multiaxial strain.7
In addition to producing and maintaining the structural components of the ECM, ligament cells produce signaling molecules that help promote formation of ACL-appropriate ECM, including proteoglycans such as decorin and glycoproteins, and members of the tenascin and thrombospondin families.8–11 Various matrix metalloproteinases (MMP) produced by ligament cells play an important role in ECM repair and remodeling.12–14 To date, no readily accessible in vivo niche has been identified, from which ligament cells can be harvested in quantities sufficient to generate engineered tissue replacements. The development of methods for deriving ligament cells from more abundant stem cell sources is therefore paramount.
Although standard protocols have been developed to differentiate mesenchymal stem cells (MSC) into other musculoskeletal cell types, such as myocytes and osteoblasts, there are no such protocols for deriving ligament cells. The most well-characterized methods use MSC from various sources, including bone marrow, adipose tissue, and stem cell lines such as C3H10T1/2 cells, cultured in a variety of three-dimensional (3D) matrices to simulate the in vivo environment.15–17 While the exact methods vary, 3D culture of MSC has been reported to upregulate tendon/ligament markers compared with monolayer culture.18 Cyclic mechanical strain further enhances the effect of 3D culture on MSC differentiation.17,18 Despite success demonstrating the importance of mechanical stimulation for tendon/ligament cell differentiation, no method produces cells capable of fully recreating the architecture and biomechanical properties of native ACL.
During early embryonic development, tendon and ligament precursor cells are defined by expression of the basic helix–loop–helix (bHLH) transcription factor, scleraxis (Scx).19,20 bHLH transcription factors play crucial roles in tissue development, often by initiating and driving cell differentiation through upregulation of tissue-specific genes.21 The classic example of bHLH transcription factors as “master regulators” of tissue development is the muscle-specific transcription factor MyoD, which, when exogenously overexpressed in C3H10T1/2 cells, induces myoblast conversion.22
In tendon, Scx plays a role in regulating the expression of several key marker genes, including the collagen type I alpha 1 (COL1A1) gene and tenomodulin (TNMD).23,24 Forced overexpression of Scx by viral transduction upregulates TNMD, increases soluble collagen production by MSC, and results in tendon-like matrix organization.25,26 These studies suggest that Scx is capable of directing tendon and ligament cell differentiation. While these results are promising, the use of viral vectors precludes translation to clinical applications due to the potential immunogenicity of virally transduced cells and the risk of tumor formation.27,28 A gene therapy method that allows significant and nonintegrative overexpression of Scx would be of great benefit in deriving mature ligament cells from MSC for use in tissue engineering.
The objective of this study was to investigate whether transient Scx overexpression in combination with culture using a 3D collagen hydrogel model is sufficient to drive ligament cell differentiation. We hypothesized that overexpression of Scx would increase expression of ligament-related genes, stimulate GAG production and matrix reorganization, and improve the mechanical properties of hydrogel constructs. We further hypothesized that addition of cyclic mechanical strain would potentiate this effect.
Materials and Methods
Cell culture
A murine MSC line (C3H10T1/2, passage 12–14; Clone 8, Animal Type Culture Collection) was expanded under standard culture conditions (37°C, 5% CO2, 90% humidity) in expansion medium (DMEM 4.5 g/L glucose, 10% FBS, l-glutamine, penicillin, and streptomycin) and seeded at 2000 cells/cm2. Medium was changed every 3 days and cells were passaged upon reaching 70% confluence.
Scleraxis overexpression
C3H10T1/2 cells were transfected (Nucleofector™ system; Lonza) with an expression vector containing the full-length murine SCX cDNA (pcDNA3.1/SCX; a gift from V. Léjard, Cordeliers Biomedical Institute, Paris, France)23 or β-galactosidase (LacZ) as a control (pcDNA3.1/LacZ; Life Technologies). This particular Scx plasmid was chosen based on its use in a previous study by Léjard et al. demonstrating that exogenous Scx expressed by this plasmid is able to transactivate the COL1A1 promoter in both COS-7 and NIH3T3 cells in the same manner as endogenous Scx in murine tenocytes.23
Cells were resuspended at 1 × 106 cells/100 μL in Nucleofector Cell Line Solution V (Lonza) containing 8 ng of plasmid and nucleofected using the T20 program. Cells were recovered in expansion medium for 15 min at 37°C before plating at 2000 cells/cm2 in six-well plates. Transfection efficiency was evaluated in LacZ expressing cells using β-galactosidase staining at 18 h postnucleofection (β-Gal Staining Kit; Invitrogen).
To assess the duration and magnitude of transient Scx overexpression, monolayers were collected every 2 h for 18 h, and then at 24, 48, and 72 h postnucleofection for SCX gene expression. To evaluate changes in cell proliferation due to Scx overexpression, separate cell monolayers were harvested every 24 h for 6 days for DNA quantification. Before generation of hydrogel constructs at 18 h postnucleofection, monolayer samples were collected for Scx protein production by western blot.
Western blotting
Eighteen hours postnucleofection, 1 × 106 cells were lysed in 500 μL of lysis buffer containing protease inhibitor cocktail (cOmplete™; Roche) using a 27-gauge needle to produce a whole cell lysate. To investigate subcellular localization of the Scx protein, whole cell lysate was centrifuged at 300 g at 4°C for 15 min. The supernatant was removed (cytosolic fraction) and the resulting pellet resuspended in lysis buffer and briefly sonicated (nuclear fraction). Whole cell lysate and cytosolic and nuclear fractions were quantified by BCA assay, heat denatured, loaded on precast 15% Tris-HCl polyacrylamide gels (Criterion™; Bio-Rad), and separated under reducing conditions before transfer to 0.45 μm PVDF membranes.
Scx was detected using a polyclonal rabbit antibody against Scx (SCXA, Catalog No. AP10439b; Abgent) and the Odyssey® CLx near-infrared fluorescent imaging system (LI-COR).
3D culture in collagen hydrogels
To improve cell viability before hydrogel fabrication, cells were allowed to recover in monolayer for 18 h before being trypsinized, pelleted, and resuspended at 1 × 106 cells/mL of neutralized collagen solution (pH 7.4–7.6) consisting of 70% (v/v) bovine collagen type I (3 mg/mL, PureCol®; Advanced BioMatrix), 20% (v/v) buffered 5 × DMEM (pH 9.0), and 10% (v/v) FBS. Collagen/cell solution (200 μL) was pipetted into each trough created in Tissue Train® culture plates using Trough Loaders™ (Flexcell International) and allowed to polymerize at 37°C, 5% CO2, and 90% humidity for 3 h before hydrogel constructs were covered with differentiation medium (expansion medium supplemented with ascorbic acid and HEPES).
Constructs were assigned to one of two experimental groups 24 h postfabrication: static or cyclic strain. Static constructs were left to generate static tension between the fixed tethers for 3, 7, or 14 days. Cyclically strained constructs were subjected to uniaxial cyclic strain (1%, 1 Hz, 30 min/day) for 7 or 14 days using the Tissue Train system.18,29 To quantify matrix reorganization, constructs were digitally imaged at 3, 7, and 14 days and construct width measured at the midpoint (ImageJ; National Institutes of Health).
Thirty minutes after completion of the final strain cycle, constructs were collected for gene expression (TRIzol® Reagent; Invitrogen) or biochemical analysis (cOmplete protease inhibitor cocktail) as described below. Duplicate constructs for gene expression and biochemical assays were combined for analysis. Three individual constructs from each group were collected for both histological evaluation and tensile testing. The entire experiment was repeated three times.
Gene expression
Total RNA isolated by column purification according to manufacturer's instructions (Direct-zol MicroPrep; Zymo Research) was evaluated spectrophotometrically for quantity and purity. First-strand cDNA was synthesized using random primers (High-Capacity cDNA Reverse Transcription Kit; Applied Biosystems). Murine-specific primer pairs for ligament-related genes of interest (Table 1) were designed (Primer Express®; Applied Biosystems), validated against known templates for primer efficiency and melt curve analysis, and used for quantitative polymerase chain reaction (SYBR Select Master Mix, Life Technologies; 7300 Real-Time PCR System, Applied Biosystems). Gene expression was calculated using the delta-delta Ct method (housekeeping gene glyceraldehyde 3-phosphate dehydrogenase and normalized to appropriate LacZ controls).30,31
Table 1.
Murine Primers Used for Quantitative Polymerase Chain Reaction
| Gene | Forward (5′–3′) | Reverse (5′–3′) | Reference |
|---|---|---|---|
| GAPDH | ATTGTGTCCGTCGTGGATCTGA | AGATGCCTGCTTCACCACCTTCTT | NM_001289726.1, exons 5–6 |
| Scleraxis | GACGGCGGCGAGAACAC | CACGGTCTTTGCTCAACTTTCTCT | NM_198885.3, exons 1–2 |
| Tenomodulin | GGCCTTAACTCTAATTGTCCTGTTTT | CTCGCCGTTGCTGTAGAAAGT | NM_022322.2, exons 2–3 |
| Collagen type Iα1 | ATGTTCAGCTTTGTGGACCT | CAGCTGACTTCAGGGATGT | NM_007742.4, exons 1–2 |
| Collagen type IIIα1 | CACCCTTCTTCATCCCACTCTTA | TCTAGACTCATAGGACTGACCAAGGT | NM_009930.2, exons 1–2 |
| Decorin | TCGAGTGGTGCAGTGTTCTGA | TTGCAGGTCTAGCAAGGTTGTGTC | NM_001190451.2, exons 2–3 |
| Tenascin-C | CCACCTAGTACTGATTTCATTGTCT | CCGTCTGGAGTGGCATCTG | NM_011607.3, exons 14–15 |
| Cartilage oligomeric matrix protein | TCCAAGAAGAATGACGATCAGAAA | CGTATTCGGTCGCCATCTATG | NM_016685.2, exons 10–11 |
| Thrombospondin-4 | CACCCCAGGTCTTTGATCTTCT | GAAGGTGGAGATGAGATAGACTTCGT | NM_011582.3, exons 1–2 |
| MMP-2 | TCACATACAGGATCATTGGTTACACA | GGCCCGAGCAAAAGCAT | NM_198885.3, exons 2–3 |
| MMP-13 | CAGTTCCAAAGGCTACAACTTGTTT | GTCCTTGGAGTGATCCAGACCTA | NM_008607.2, exons 4–5 |
| Alkaline phosphatase | CTTGACTGTGGTTACTGCTGATCA | GCCAGACCAAAGATGGAGTTG | NM_007431.2, exons 4–5 |
| Osteocalcin | CTGGCTGCGCTCTGTCTCT | GACATGAAGGCTTTGTCAGACTCA | NM_007541.2, exons 2–3 |
| Collagen type IIα1 | AAGTCACTGAACAACCAGATTGAGA | AAGTGCGAGCAGGGTTCTTG | NM_031163.3, exon 51 |
| Aggrecan | GCATGAGAGAGGCGAATGGA | GCTGATCTCGTAGCGATCTTTCTT | NM_007424.2, exons 15–16 |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MMP, matrix metalloproteinases.
Biochemical analysis
Constructs for GAG and DNA content were lyophilized and digested in 0.5 mg/mL papain (Sigma-Aldrich Corporation) for 4 h at 65°C. GAG content was measured using the dimethylmethylene blue dye-binding assay.32,33 Samples were digested for an additional 16 h at 65°C for DNA quantification using the bisbenzamide fluorometric assay.33,34 Values are reported as the average (μg) per construct.
Histology
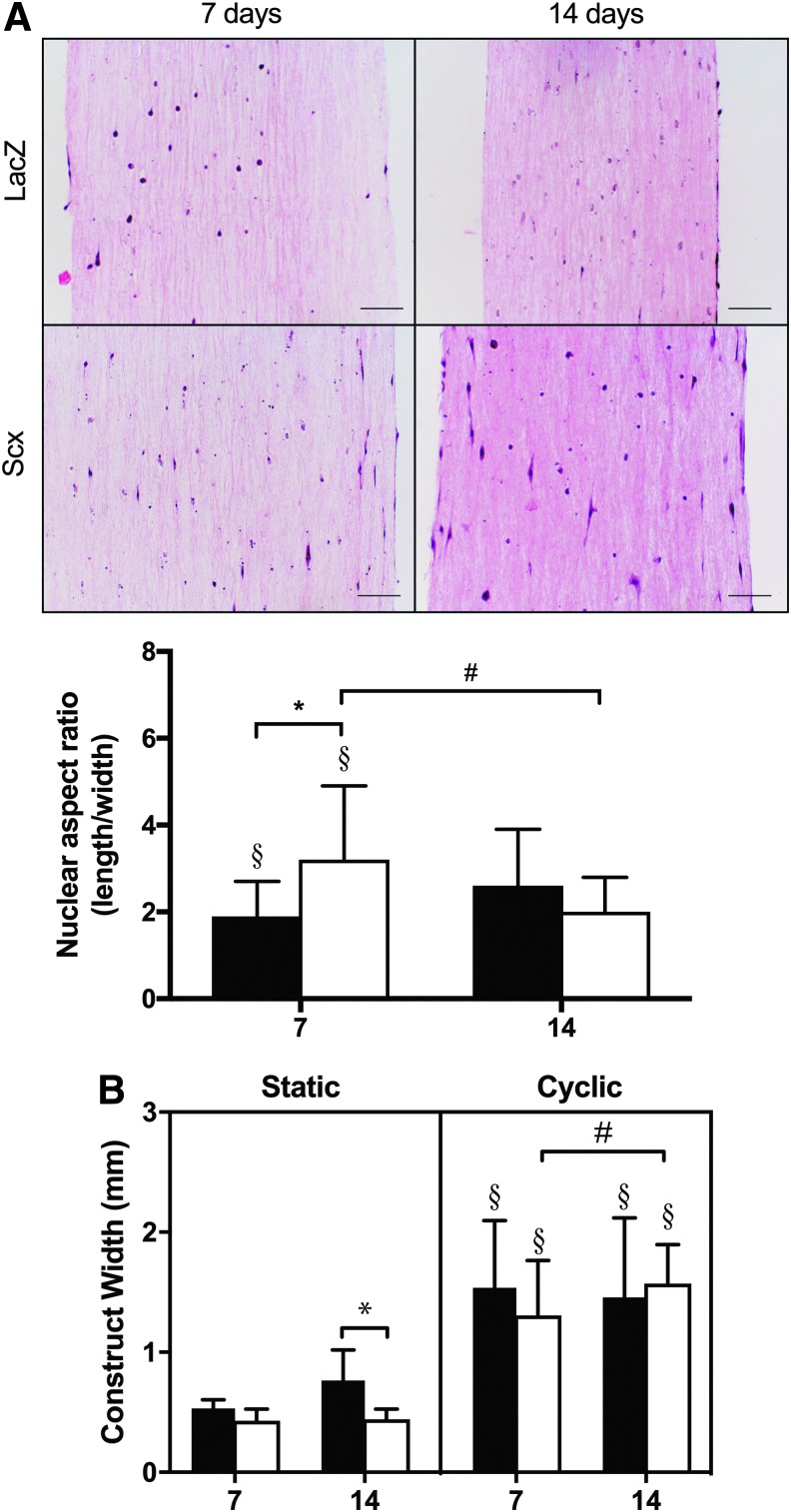
Constructs were rinsed in Dulbecco's phosphate-buffered saline (DPBS), fixed in situ in the Tissue Train plates in 4% paraformaldehyde for 30 min at room temperature, rinsed, and serially dehydrated in ethanol and xylene before paraffin embedding and sectioning at 10 μm. Sections were stained with Harris hematoxylin and eosin (H&E) and imaged (Vanox AHMT microscope; Olympus) using commercial software (DP Controller; Olympus). To assess the degree of cell alignment, five fields were imaged at 40 × from the middle region of each construct. Nuclear aspect ratio was calculated as the longest length of the nucleus divided by the perpendicular width of the same nucleus (ImageJ) for at least five cells per field.
Tensile testing
After the final strain cycle on days 7 and 14, strained constructs and their static controls were left attached to the embedded tethers, removed from the plates, and used for single load to failure tensile testing (4411 Tensile Tester; Instron). Constructs were loaded into the test grips using the tethers to minimize construct damage and preloaded to 14 mm. Each construct was strained at a rate of 1% elongation per minute (1.5 mm/min). Force and displacement data were collected to generate smoothed stress-strain curves (SigmaPlot 12.0; Systat Software). Maximum load was defined as the highest point on the curve and stiffness was calculated as the slope of the most linear portion of the curve (r2 ≥ 0.99).
Statistical analysis
For characterization of Scx overexpression in monolayer culture, differences in gene expression and DNA content between LacZ- and Scx-overexpressing cells were evaluated by Student's t-test. For data generated from constructs in 3D culture (gene expression, GAG/DNA content, and tensile testing), three-way ANOVAs were performed to investigate potential interactions between Scx overexpression and mechanical strain over time. Fixed effects with p ≤ 0.1 were considered for further investigation.
To compare specific groups within each effect level, the slicdiff option of proc glimmix was used. Model fit was determined by examining studentized residual plots. Values for relative gene expression in 3D culture were log transformed to stabilize the model. A simple effect p-value of ≤0.05 was considered statistically significant. All data are shown as mean ± SD. All analyses were performed in SAS Studio 3.6 (SAS Institute, Inc.).
Results
Scleraxis overexpression in monolayer culture
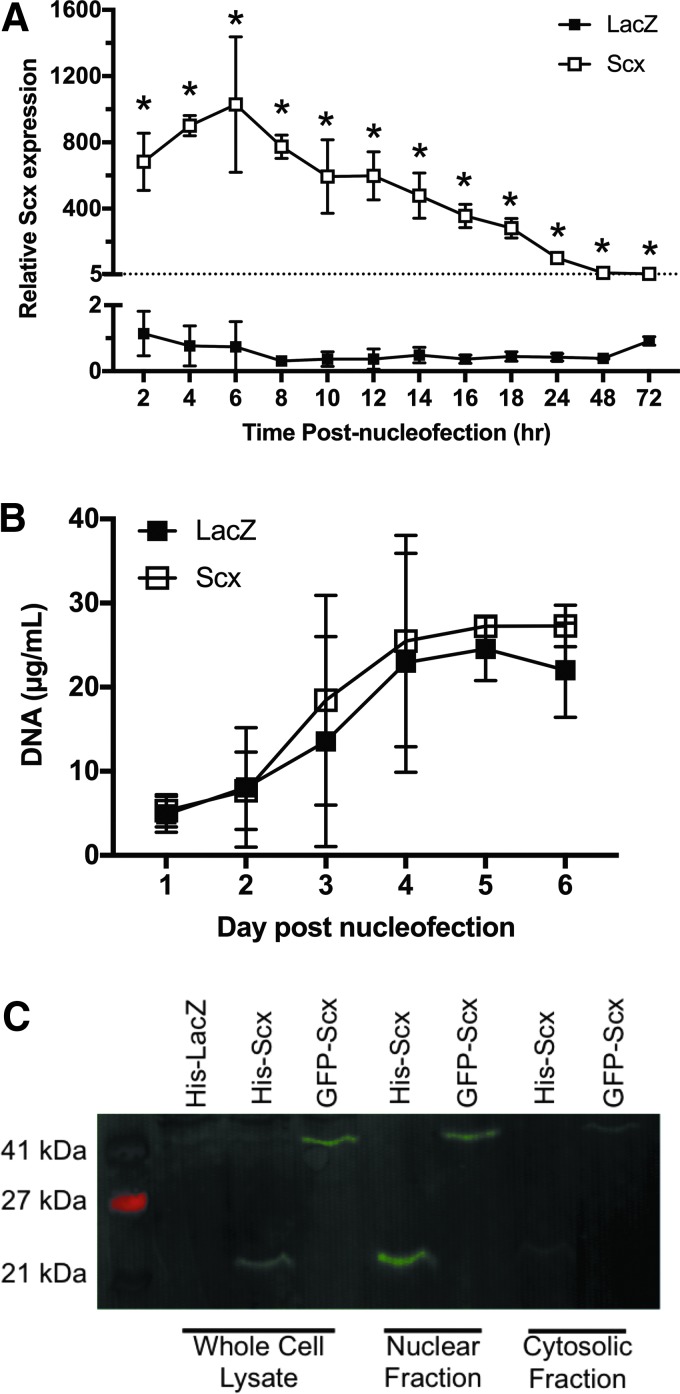
Transfection efficiency using the described protocol was ∼80% at 18 h postnucleofection based on LacZ staining. SCX gene expression was significantly increased compared to LacZ controls at all time points up to 72 h following nucleofection (p < 0.01 for all time points; Fig. 1A). There was no difference in the DNA content of Scx-overexpressing and LacZ cell monolayers over 6 days (p = 0.447; Fig. 1B). Scx protein was increased in Scx-overexpressing cells compared to LacZ and was located mainly in the nuclear fraction of the cell lysate (Fig. 1C).
FIG. 1.
Effects of scleraxis (Scx) overexpression in C3H10T1/2 cells in monolayer culture. (A) Scx overexpression resulted in significantly increased SCX gene expression for at least 72 h compared to LacZ controls (n = 3). (B) DNA content increased significantly over time in both groups (n = 3; p = 0.009); however, there was no difference between LacZ and Scx cells. (C) Overexpressed His-Scx protein (∼23 kDa) was localized in the nuclear fraction, indicating proper vector function and nuclear translocation. GFP-Scx (49 kDa) was used as an antibody control. *Significant changes relative to LacZ (p < 0.05). LacZ, β-galactosidase; Scx, scleraxis.
Effects of static 3D culture in collagen hydrogels
ECM organization
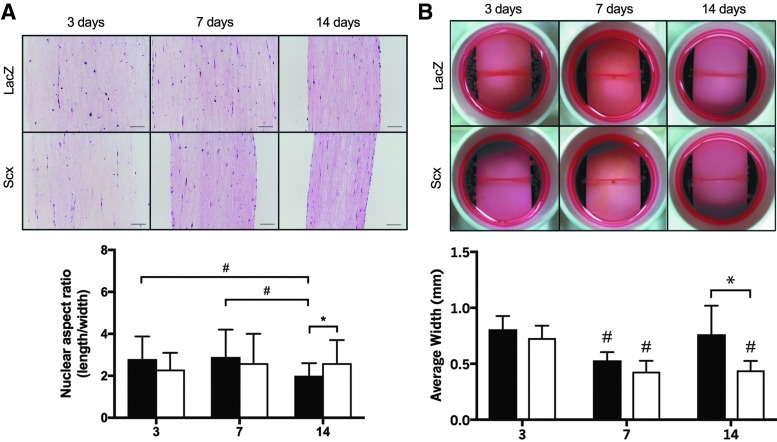
LacZ-expressing cells in static constructs became less elongated over time, with rounder nuclei at 14 days compared with both 3 (p = 0.005) and 7 days (p = 0.002; Fig. 2A). In contrast, Scx-overexpressing cells remained elongated along the length of the construct throughout the experiment, and at 14 days had significantly more elongated nuclei compared to LacZ cells (p = 0.031). No overt differences in matrix staining or ECM organization between LacZ and Scx constructs were observed at any time point (Fig. 2A).
FIG. 2.
Histological effects of Scx overexpression in C3H10T1/2 cells cultured in static 3D collagen hydrogels. (A) Representative H&E stained constructs over 14 days in culture. Scale bar = 200 μm. LacZ cells became rounder over time, whereas cells overexpressing Scx remained elongated (n = 25–40 cells). (B) Constructs containing Scx-overexpressing cells were significantly narrower than those containing LacZ cells at 14 days (n = 18). #Significant effect of time or *gene (p < 0.05). 3D, three-dimensional; H&E, hematoxylin and eosin.
Both time (p < 0.001) and Scx overexpression (p = 0.043) had an effect on construct width in static culture (Fig. 2B). LacZ constructs contracted from 3 to 7 days (p = 0.043), but returned to their 3-day width by 14 days (p = 0.919). Scx constructs also contracted significantly from 3 to 7 days (p = 0.026); however, they remained narrower at 14 days than at 3 days (p = 0.034). Scx constructs were significantly narrower than LacZ constructs after 14 days of static culture (p = 0.006).
Biochemical composition
There were no differences in DNA content between Scx-overexpressing constructs and LacZ controls under static conditions (p = 0.175); however, there was an effect of time on DNA content (p < 0.001). DNA decreased significantly between 3 and 7 days (p = 0.013) and from 3 to 14 days (p = 0.006) in Scx-overexpressing constructs (Table 2). A similar, although not significant, decrease in DNA content was seen in LacZ constructs, with less DNA at 14 days compared to 3 days (p = 0.093). There was no effect of either Scx overexpression (p = 0.767) or time (p = 0.310) on overall GAG content of constructs (Table 2).
Table 2.
DNA and Glycosaminoglycan Content of Static and Cyclic Constructs
| DNA content (μg/construct) | GAG content (μg/construct) | |||
|---|---|---|---|---|
| Static | LacZ | Scx | LacZ | Scx |
| 3 days | 54.57 ± 14.66 | 65.99 ± 22.19 | 8.29 ± 3.74 | 7.38 ± 1.22 |
| 7 days | 35.32 ± 10.59 | 38.08 ± 10.59a | 5.72 ± 0.82 | 6.69 ± 1.04 |
| 14 days | 36.05 ± 13.69 | 36.79 ± 5.56a | 6.16 ± 2.63 | 6.08 ± 1.24 |
| Cyclic | LacZ | Scx | LacZ | Scx |
| 7 days | 33.47 ± 1.93 | 35.86 ± 3.69 | 7.53 ± 0.42 | 7.32 ± 1.27 |
| 14 days | 29.16 ± 3.22 | 35.99 ± 10.64 | 7.89 ± 3.82 | 9.70 ± 3.12b |
Significant change from day 3 static constructs (within gene group) at p < 0.05.
Significant change compared to static (same gene and day) at p < 0.05.
GAG, glycosaminoglycan; LacZ, β-galactosidase; Scx, scleraxis.
Gene expression
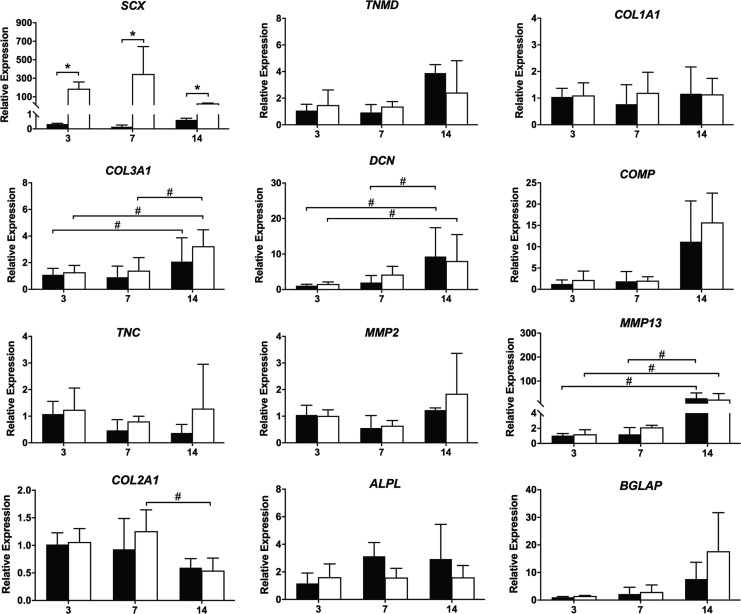
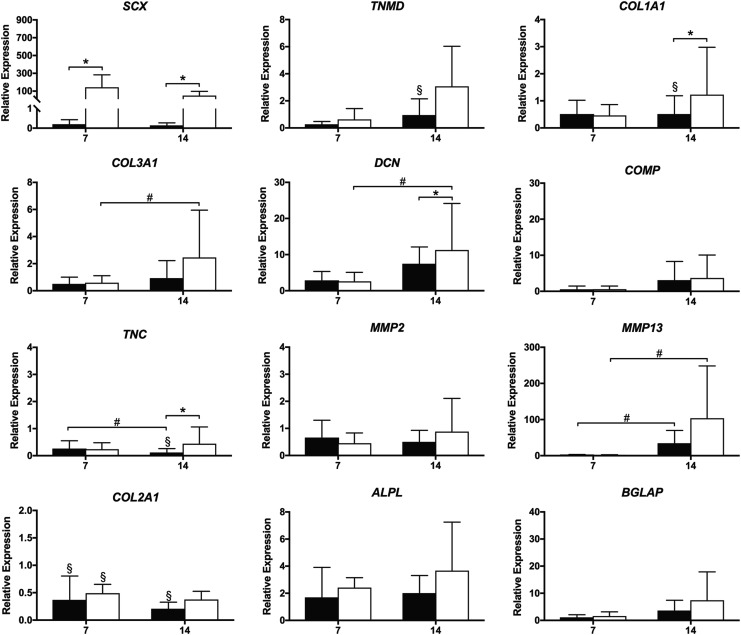
There was a two-way interaction between Scx overexpression and time on Scx expression (p = 0.031; Fig. 3). SCX expression was nearly 200-fold higher in Scx constructs compared to LacZ controls at 3 days (p < 0.001) and peaked at 7 days (300-fold compared to LacZ; p < 0.001) before decreasing. At 14 days, SCX expression remained significantly elevated compared to LacZ controls (30-fold; p = 0.006). SCX expression also increased in static LacZ constructs from 7 to 14 days, although this increase was not statistically significant (p = 0.086). Aggrecan and thrombospondin-4 were below detectable limits in all samples. There were no other significant effects of Scx overexpression compared to LacZ controls on any of the ligament-related genes examined.
FIG. 3.
Ligament-related gene expression over 14 days in C3H10T1/2 cells in static 3D culture. Black bars = LacZ; white bars = Scx. Transcripts were normalized to GAPDH and 3 day LacZ constructs (n = 3). #Significant effect of time or *gene (p < 0.05 unless otherwise stated). ALPL, alkaline phosphatase; BGLAP, bone gamma-carboxyglutamate protein (osteocalcin); COL1A1, collagen type Iα1; COL2A1, collagen type II; COL3A1, collagen type IIIα1; COMP, cartilage oligomeric matrix protein; DCN, decorin; MMP2, matrix metalloproteinase metalloproteinase-2; MMP13, matrix metalloproteinase metalloproteinase-13; TNC, tenascin-C; TNMD, tenomodulin.
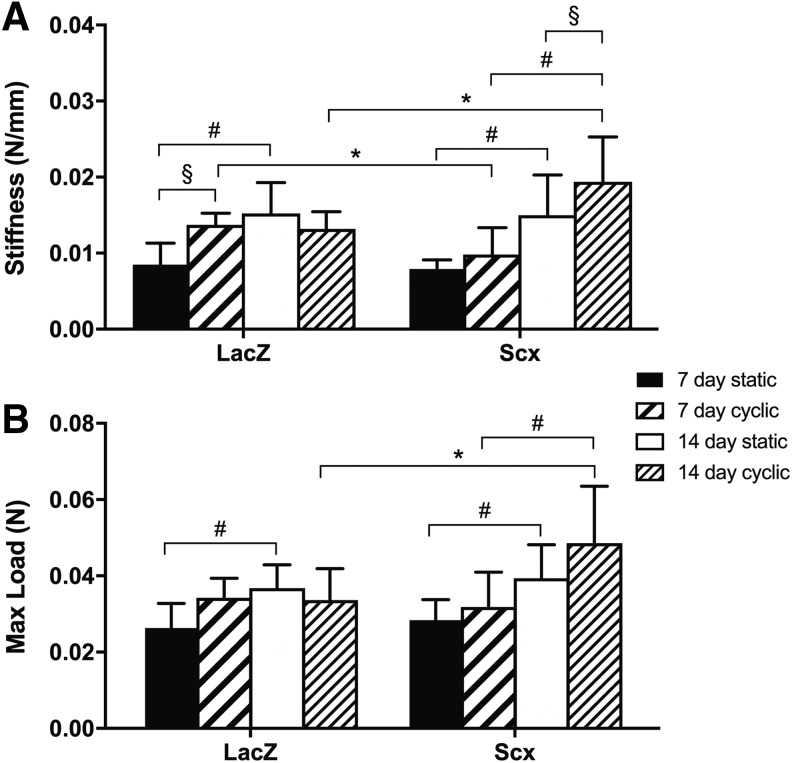
Mechanical properties
The stiffness of LacZ- and Scx-overexpressing constructs nearly doubled from 7 to 14 days (p < 0.001 for both; Fig. 4A). There was no difference in stiffness between LacZ and Scx-overexpressing constructs at 7 or 14 days (p = 0.76 and p = 0.944, respectively; Fig. 4A). Maximum load of both groups increased ∼40% from 7 to 14 days (LacZ p = 0.008; Scx p = 0.006; Fig. 4B) with no differences between LacZ- and Scx-overexpressing constructs at 7 (p = 0.615) or 14 days (p = 0.541).
FIG. 4.
Mechanical properties of 3D collagen hydrogels containing C3H10T1/2 cells. In static constructs, stiffness (A) and maximum load (B) increased in all constructs from 7 to 14 days, regardless of Scx overexpression (n = 9). In cyclically strained constructs, the stiffness and maximum load of Scx constructs increased from 7 to 14 days, whereas mechanical properties of LacZ constructs were unchanged. At 14 days, Scx constructs had a higher maximum load and were stiffer than LacZ controls (n = 9). #Significant effect (p < 0.05) of time, *gene, or §strain.
Effects of cyclic mechanical strain in 3D collagen hydrogels
ECM organization
There was a three-way interaction among Scx overexpression, time, and cyclic strain (p < 0.001) and a two-way interaction between Scx overexpression and time (p = 0.076) on the nuclear aspect ratio of cells in 3D constructs (Fig. 5A). The nuclei of Scx-overexpressing cells in strained constructs were more elongated compared with static constructs at 7 and 14 days (p = 0.031 and p = 0.064, respectively). At 7 days, cyclically strained Scx-overexpressing nuclei were significantly more elongated compared to LacZ (p < 0.001). Strained LacZ nuclei were rounder than static LacZ cells at 7 days (p = 0.002), but more elongated compared to static LacZ nuclei at 14 days (p = 0.041).
FIG. 5.
Effects of cyclic mechanical strain on C3H10T1/2 cells in 3D collagen hydrogels. (A) Representative H&E stained construct over 14 days in culture. Scale bar = 200 μm. Scx-overexpressing cells became more elongated with cyclic strain at 7 days, whereas LacZ were more rounded (n = 25–40 cells). (B) Both Scx-overexpressing and LacZ constructs were wider than their static counterparts (n = 18). Black bars = LacZ; white bars = Scx. #Significant effect (p < 0.05) of time, *gene, or §strain.
There was a three-way interaction among Scx overexpression, time, and cyclic mechanical strain on the width of constructs (p = 0.028). Strain also had an effect on the width of constructs (p < 0.001). LacZ and Scx constructs were significantly wider than their static counterparts at 7 (p < 0.001 for both) and 14 days (p < 0.001 for both; Fig. 5B). There were no changes in LacZ construct width over time with cyclic strain (p = 0.532). In contrast, the width of strained Scx constructs increased significantly from 7 to 14 days (p = 0.039), but was not significantly different compared with LacZ constructs at either 7 (p = 0.073) or 14 days (p = 0.371).
Biochemical composition
The addition of cyclic strain had an effect (p = 0.043) on GAG production, with significantly higher levels of GAG in Scx constructs compared to static culture at 14 days (p = 0.042; Table 2); however, there was no difference between Scx and LacZ constructs (p = 0.295). There was no effect of cyclic strain on DNA content (p = 0.493; Table 2).
Gene expression
Scx expression remained significantly elevated in Scx-overexpressing constructs at 7 and 14 days (p < 0.001) compared to LacZ constructs, but was not affected by the addition of cyclic strain in either Scx-overexpressing constructs or LacZ controls (p = 0.599; Fig. 6). There was a three-way interaction among Scx overexpression, time, and cyclic strain (p = 0.071) and a two-way interaction between Scx overexpression and cyclic strain on the expression of TNMD (p = 0.091). TNMD was significantly decreased in strained LacZ constructs compared to static at 14 days (p = 0.028), whereas TNMD expression was unaffected by cyclic strain in Scx constructs at either 7 or 14 days (p = 0.229 and p = 0.217, respectively).
FIG. 6.
Ligament-related gene expression over 14 days in C3H10T1/2 cells in cyclic 3D culture. Black bars = LacZ; white bars = Scx. Transcripts were normalized to GAPDH and 3 day static LacZ constructs (n = 3). §Significant effect (p < 0.05) of cyclic strain (compared to static), #time, or *gene.
There was a three-way interaction among Scx overexpression, time, and cyclic strain (p = 0.077) and two-way interaction between Scx overexpression and cyclic strain (p = 0.073) on the expression of COL1A1. COL1A1 expression decreased significantly in strained LacZ constructs from 3 to 14 days (p = 0.005) and at 14 days was significantly decreased compared with static LacZ constructs (p = 0.005). Strained Scx constructs had significantly increased COL1A1 expression compared with strained LacZ constructs at 14 days (p = 0.002); however, COL1A1 expression in Scx-overexpressing constructs was not different between static and strained groups (p = 0.275).
There were no two- or three-way interaction effects on the expression of collagen type III alpha 1 (COL3A1); however, COL3A1 expression increased over time in strained Scx constructs from 7 to 14 days (p = 0.003), although this increase was not significantly different from LacZ (p = 0.445). There was a three-way interaction among Scx-overexpression, time, and cyclic strain on the expression of decorin (DCN) (p = 0.066). In strained Scx constructs, DCN expression increased from 7 to 14 days (p = 0.008) and at 14 days was higher compared with LacZ constructs (p = 0.037). DCN expression did not change over time in LacZ constructs (p = 0.368) and tended to be lower than strained Scx constructs at 14 days (p = 0.091).
In strained LacZ constructs, tenascin-C (TNC) expression decreased significantly from 7 to 14 days (p = 0.05) and at 14 days was significantly lower than static LacZ constructs (p = 0.018) and strained Scx constructs (p = 0.022). The addition of cyclic strain had an effect on expression of the cartilage marker, collagen type II alpha 1 (COL2A1; p = 0.001), with decreased expression in strained Scx constructs at 7 days (p = 0.033) and in LacZ constructs compared with static at 7 (p = 0.045) and 14 days (p = 0.013).
There was a two-way interaction between Scx overexpression and cyclic strain on expression of the osteoblast marker alkaline phosphatase (ALPL; p = 0.064) with decreased expression in strained LacZ constructs compared to static at 7 days (p = 0.068). Cyclic strain also had an effect on expression of the osteoblast marker osteocalcin (BGLAP; p = 0.098), with decreased expression in strained Scx constructs compared with static Scx constructs at 14 days, (p = 0.086), although this change was not statistically significant.
Mechanical properties
There was a three-way interaction between Scx overexpression, time, and cyclic strain on both the stiffness (p = 0.025) and maximum load (p = 0.059) of collagen constructs. After 7 days of cyclic strain, Scx constructs were ∼30% less stiff than LacZ constructs (p = 0.036; Fig. 4A). The stiffness of strained Scx constructs nearly doubled from 7 to 14 days (p < 0.001), and at 14 days Scx constructs were significantly stiffer than both strained LacZ controls (∼46%; p = 0.009) and static Scx constructs (∼30%; p = 0.033). In contrast, strained LacZ constructs were ∼60% stiffer than static constructs at 7 days (p = 0.005), but did not increase in stiffness from 7 to 14 days (p = 0.967), and were not different from their static counterparts at 14 days (p = 0.553).
A similar pattern was observed for the maximum load of strained constructs (Fig. 4B). The maximum load of strained Scx constructs increased ∼50% over time from 7 to 14 days (p < 0.001), and at 14 days, strained Scx constructs had a significantly higher maximum load compared with strained LacZ constructs (∼45%; p = 0.001). The maximum load of LacZ constructs did not change over time from 7 to 14 days (p = 0.862). Both strained LacZ and Scx constructs had a higher maximum load compared with their static counterparts, with increases in LacZ constructs at 7 days (p = 0.054) and in Scx constructs at 14 days (p = 0.068); however, these changes failed to reach statistical significance.
Discussion
Despite its frequent use as an early marker of ligament cell identity and the important role of other bHLH transcription factors in driving tissue development, the use of Scx to induce ligament cell differentiation in stem cells for tissue engineering remains unexplored. Using nucleofection, we were able to achieve significantly increased and sustained expression of Scx for the 14 days of our study. When cultured in static 3D collagen hydrogel constructs, Scx-overexpressing cells were more elongated and better able to contract the surrounding matrix compared to LacZ control cells.
With the addition of cyclic strain, Scx-overexpressing cells maintained expression of the ligament marker genes COL1A1, DCN, and TNC, and improved the overall mechanical properties of collagen constructs compared to LacZ. Based on the results of this study, transient Scx overexpression in combination with cyclic mechanical strain may be a useful means of driving ligamentous differentiation of MSC for ligament engineering studies.
Cells in monolayer culture demonstrated a 200-fold increase in SCX expression compared to LacZ controls 18 h postnucleofection; however, SCX expression decreased to approximately fourfold after 72 h. In contrast, Scx-overexpressing cells cultured in 3D constructs maintained SCX expression (at least 30-fold) for 14 days under both static and cyclic conditions.
Both 3D culture and cyclic strain have been reported to upregulate endogenous SCX expression, although the exact mechanism by which this occurs remains unclear.17,18 SCX is regulated, in part, by members of the transforming growth factor superfamily via Smad2/335 and can in turn facilitate Smad3-mediated signaling by promoting the formation of transcriptional complexes.36 As Smad2/3 serves as a critical node mediating both biochemical and mechanical induction of Scx in tenocytes, these data suggest Scx may promote its own expression through a positive feedback mechanism.35
In our study, exogenous expression of SCX may have “primed” Smad2/3-mediated signaling, resulting in increased endogenous SCX expression with the introduction of mechanical strain. This supports the idea that transient Scx overexpression may be sufficient to initiate the ligamentous differentiation process through activation of a positive feedback loop, although the contribution of endogenous versus exogenous Scx and the underlying mechanism require further confirmation.
Scx overexpression combined with static 3D culture resulted in elongated cells and narrower constructs after 14 days, suggesting a greater capacity for matrix reorganization in cells overexpressing Scx compared to LacZ controls. The uniquely anisotropic and highly organized nature of ligament ECM is vital in providing tensile strength to ligament and in promoting normal function of resident cells, and vice versa. Cells embedded within healthy ligament are defined by their alignment parallel to collagen fibrils and their ability to actively remodel the surrounding matrix.2 Greater cell elongation in Scx-overexpressing constructs is therefore consistent with greater contraction, and together these observations are indicative of a more differentiated cell phenotype.
Despite this, there were no concurrent increases in ligament-related gene expression, GAG production, or overall mechanical properties due to Scx overexpression in static culture, suggesting that Scx alone is insufficient to induce robust cell differentiation. With the addition of cyclic strain, Scx-overexpression prevented the strain-induced decrease in expression of the ligament marker genes COL1A1, DCN, and TNC, rounder cell phenotype, and decreased tensile strength of LacZ constructs. Contrary to our hypothesis, overexpression of Scx in combination with cyclic strain did not have an additive effect on expression of ligament-related genes; instead, it appears that Scx overexpression may amplify the proligamentous effect of otherwise insufficient cyclic strain levels.
Strained LacZ and Scx constructs were wider and had modest increases in GAG content compared to their static counterparts. GAGs contribute to tissue hydration by attracting and binding water molecules due to their large surface area and highly negative charge, and tissue hydration state affects the viscoelastic behavior of ligament.37,38 Increased width in cyclically strained constructs could be attributed to construct swelling due to increased GAG content. This is in contrast to reports demonstrating similar contraction rates between static and cyclically strained constructs, although differences in cell type and strain regimen could account for this inconsistency.17,18
Despite similar increases in construct width and GAG content as strained LacZ constructs, strained Scx constructs exhibited increased overall tensile strength compared with both static and strained LacZ constructs. Interestingly, we were unable to correlate this observation to any of the parameters measured. With Scx overexpression and cyclic strain, we detected no associated increases in genes indicative of catabolic remodeling (MMP2, MMP13) and only a modest increase in expression of anabolic response genes (COL1A1, COL3A1, DCN, TNC) that was not different from static culture. This suggests that Scx promotes ligament organization by a mechanism other than bulk synthesis of ECM.
A study by Marturano et al. reported that changes in collagen cross-linking can alter the mechanical properties of embryonic tendons without affecting collagen matrix composition or structure.39 Studies aimed at evaluating how Scx may regulate the expression or activity of various collagen cross-linking enzymes, such as lysyl oxidase, are needed to further define the role Scx may play in facilitating ECM organization.
To our knowledge, only one other study has investigated the combined effects of Scx overexpression and mechanical stimulation on MSC tendon/ligament differentiation.26 Using a lentiviral vector to overexpress Scx in human embryonic stem cell-derived MSC and a scaffold-free 3D culture system, Chen et al. reported upregulation of the tendon markers COL1A1 and TNMD and modest improvements in ECM organization with Scx overexpression and mechanical strain, but not with Scx overexpression alone. This observation is consistent with our findings. Chen et al. also reported that in vitro cell differentiation was greatly enhanced by implantation in an in vivo model.
It is possible that our method would show similar results in an in vivo model. Our results using a transient, nonviral method of Scx upregulation are similar to the viral method, which is encouraging and suggests that our method is a viable and useful alternative. The results of both studies point to the importance of identifying additional extrinsic factors involved in ligament and tendon tissue maturation to facilitate development of tissue replacements.
Although the ultimate source of stem cells for ligament tissue engineering is likely to be autologous patient-derived MSC, use of model stem cell lines such as C3H10T1/2 cells allows investigations on a well-characterized, homogenous cell population that is free from the phenotypic and biological heterogeneity inherent in primary cell cultures.40,41 Similar to primary MSC, C3H10T1/2 cells are able to undergo trilineage differentiation.42 Furthermore, mechanisms identified in C3H10T1/2 cells are highly translatable to primary MSC, and their use has been key in identifying and validating specific mechanisms driving the differentiation of other musculoskeletal tissues, including muscle, cartilage, and bone.22,43,44
In tendon and ligament, C3H10T1/2 cells have been used to demonstrate the effects of cyclic strain on Scx expression and to elucidate signaling pathways underlying the protenogenic effects of various growth factors.17,45 Our study therefore lays the groundwork for examination of the effects of Scx overexpression in primary MSC.
The Flexcell Tissue Train system is well established in the tendon/ligament literature and for demonstrating the protenogenic effects of cyclic strain on MSC differentiation17,18 and maintenance of primary tenocyte phenotype in vitro.29 The choice of cell type, strain magnitude, and duration makes comparisons between studies difficult. The strain protocol used in our study was based on previous studies showing a pro-tenogenic effect in adult human bone marrow MSC and avian tenocytes18,29; however, tenogenesis of C3H10T1/2 cells has been reported to be strain dependent, with higher strain levels (up to 10%) and lower frequency (0.1 Hz) resulting in increased expression of both Scx and COL1.17
We did not observe ligamentous differentiation of the LacZ control cells in our study; however, Scx-overexpression in combination with the same protocol resulted in a stiffer construct, more elongated cell phenotype, and sustained expression of ligament marker genes. This lends further support to the idea that exogenous Scx expression augmented the mechanosensing capabilities of cells, such that even our modest strain regimen improved ligament cell differentiation. Scx was recently reported to be required for the protenogenic effects of mechanical strain on MSC.45 Future studies examining the effects of various strain magnitudes in this system would help clarify the relationship between Scx and the mechanosensing ability of ligament cells.
In summary, nucleofection was an effective means of upregulating Scx at both the mRNA and protein levels and culture in 3D constructs maintained increased Scx expression for up to 14 days. Scx overexpression combined with static culture resulted in increased construct contraction, elongated cell phenotype, and more mechanically sensitive cells, as evidenced by maintenance of ligament marker genes and increased overall construct strength. Combined, these data demonstrate the potential effectiveness of transient Scx upregulation for inducing ligament differentiation and lend further support to the existence of a synergistic role for Scx and mechanical strain during early tissue development.
Acknowledgments
The authors thank Véronique Léjard, Cordeliers Biomedical Institute, Paris, France for the kind gift of the Scx plasmid. The authors also acknowledge Robyn Cardwell for her early work with the Scx plasmid, Daniel Inman and Kathryn Slaughter for their help in optimizing nucleofection protocols, and Brandon Wiese for his assistance with histological evaluation. This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (1R15AR057575-01A1). A.E.C.N. received fellowship support from the Stamps Family Charitable Foundation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Mall N.A., Chalmers P.N., Moric M., et al. . Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med 42, 2363, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Amiel D., Frank C., Harwood F., Fronek J., and Akeson W. Tendons and ligaments: a morphological and biochemical comparison. J Orthop Res 1, 257, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Kiapour A.M., and Murray M.M. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res 3, 20, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kartus J., Movin T., and Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy 17, 971, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Robertson A., Nutton R.W., and Keating J.F. Current trends in the use of tendon allografts in orthopaedic surgery. J Bone Joint Surg Br 88, 988, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Rumian A.P., Wallace A.L., and Birch H.L. Tendons and ligaments are anatomically distinct but overlap in molecular and morphological features–a comparative study in an ovine model. J Orthop Res 25, 458, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Strocchi R., de Pasquale V., Gubellini P., et al. . The human anterior cruciate ligament: histological and ultrastructural observations. J Anat 180, 515, 1992 [PMC free article] [PubMed] [Google Scholar]
- 8.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84, 649, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Benjamin M., and Ralphs J.R. The cell and developmental biology of tendons and ligaments. Int Rev Cytol 196, 85, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Zhang G., Ezura Y., Chervoneva I., et al. . Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem 98, 1436, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Chiquet-Ehrismann R., and Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol 200, 488, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Foos M.J., Hickox J.R., Mansour P.G., Slauterbeck J.R., and Hardy D.M. Expression of matrix metalloprotease and tissue inhibitor of metalloprotease genes in human anterior cruciate ligament. J Orthop Res 19, 642, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Zhou D., Lee H.S., Villarreal F., et al. . Differential MMP-2 activity of ligament cells under mechanical stretch injury: an in vitro study on human ACL and MCL fibroblasts. J Orthop Res 23, 949, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Roseti L., Buda R., Cavallo C., Desando G., Facchini A., and Grigolo B. Ligament repair: a molecular and immunohistological characterization. J Biomed Mater Res 84, 117, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Yang G., Rothrauff B.B., Lin H., Gottardi R., Alexander P.G., and Tuan R.S. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 34, 9295, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thayer P.S., Dimling A.F., Plessl D.S., et al. . Cellularized cylindrical fiber/hydrogel composites for ligament tissue engineering. Biomacromolecules 15, 75, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Scott A., Danielson P., Abraham T., Fong G., Sampaio A.V., and Underhill T.M. Mechanical force modulates scleraxis expression in bioartificial tendons. J Musculoskelet Neuronal Interact 11, 124, 2011 [PubMed] [Google Scholar]
- 18.Kuo C.K., and Tuan R.S. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng A 14, 1615, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Schweitzer R., Chyung J.H., Murtaugh L.C., et al. . Analysis of the tendon cell fate using scleraxis, a specific marker for tendons and ligaments. Development 128, 3855, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Cserjesi P., Brown D., Ligon K.L., et al. . Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development 121, 1099, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Jones S. An overview of the basic helix-loop-helix proteins. Genome Biol 5, 226, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis R.L., Weintraub H., and Lassar A.B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51, 987, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Léjard V., Brideau G., Blais F., et al. . Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem 282, 17665, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Shukunami C., Takimoto A., Oro M., and Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol 298, 234, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Alberton P., Popov C., Prägert M., et al. . Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev 21, 846, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X., Yin Z., Chen J.L., et al. . Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Sci Rep 2, 977, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouard D., Alazard-Dany D., and Cosset F.L. Viral vectors: from virology to transgene expression. Br J Pharmacol 157, 153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howarth J.L., Lee Y.B., and Uney J.B. Using viral vectors as gene transfer tools (Cell Biology and Toxicology Special Issue: ETCS-UK 1 day meeting on genetic manipulation of cells). Cell Biol Toxicol 26, 1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garvin J., Qi J., Maloney M., and Banes A.J. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng 9, 967, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Cardwell R.D., Dahlgren L.A., and Goldstein A.S. Electrospun fibre diameter, not alignment, affects mesenchymal stem cell differentiation into the tendon/ligament lineage. J Tissue Eng Regen Med 8, 937, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Livak K.J., and Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Farndale R.W., Buttle D.J., and Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883, 173, 1986 [DOI] [PubMed] [Google Scholar]
- 33.Cissell J.M., Milton S.C., and Dahlgren L.A. Investigation of the effects of prostaglandin E2 on equine superficial digital flexor tendon fibroblasts in vitro. Vet Comp Orthop Traumatol 23, 417, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Rao J., and Otto W.R. Fluorimetric DNA assay for cell growth estimation. Anal Biochem 207, 186, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Maeda T., Sakabe T., Sunaga A., et al. . Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr Biol 21, 933, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagchi R.A., Roche P., Aroutiounova N., et al. . The transcription factor scleraxis is a critical regulator of cardiac fibroblast phenotype. BMC Biol 14, 21, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thornton G.M., Shrive N.G., and Frank C.B. Altering ligament water content affects ligament pre-stress and creep behaviour. J Orthop Res 19, 845, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Chimich D., Shrive N., Frank C., Marchuk L., and Bray R. Water content alters viscoelastic behaviour of the normal adolescent rabbit medial collateral ligament. J Biomech 25, 831, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Marturano J.E., Xylas J.F., Sridharan G.V., Georgakoudi I., and Kuo C.K. Lysyl oxidase-mediated collagen crosslinks may be assessed as markers of functional properties of tendon tissue formation. Acta Biomater 10, 1370, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitfield M.J., Lee W.C.J., and Van Vliet K.J. Onset of heterogeneity in culture-expanded bone marrow stromal cells. Stem Cell Res 11, 1365, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Baer P.C. Adipose-derived mesenchymal stromal/stem cells: an update on their phenotype in vivo and in vitro. World J Stem Cells 6, 256, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao L., Li G., Chan K.M., Wang Y., and Tang P.F. Comparison of multipotent differentiation potentials of murine primary bone marrow stromal cells and mesenchymal stem cell line C3H10T1/2. Calcif Tissue Int 84, 56, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Denker A.E., Haas A.R., Nicoll S.B., and Tuan R.S. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: I. Stimulation by bone morphogenetic protein-2 in high-density Micromass cultures. Differentiation 64, 67, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Katagiri T., Yamaguchi A., Ikeda T., et al. . The non-osteogenic mouse pluripotent cell line, C3H10T1/2, is induced to differentiate into osteoblastic cells by recombinant human bone morphogenetic protein-2. Biochem Biophys Res Commun 172, 295, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Ramcharan M., Zhou Z., et al. . The role of scleraxis in fate determination of mesenchymal stem cells for tenocyte differentiation. Sci Rep 5, 13149, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]