Abstract
Research investigating multisensory integration (MSI) processes in aging is scarce, but converging evidence for larger behavioral MSI effects in older compared to younger adults exists. The current study employed a three-prong approach to determine whether inherent age-related sensory processing declines were associated with larger (i.e., worse) visual-somatosensory (VS) reaction time (RT) facilitation effects. Non-demented older adults (n = 156; mean age 77 55% female) withoutany medical or psychiatric conditions =years;were included. Participants were instructed to make speeded foot-pedal responses as soon as they detected visual, somatosensory, or VS stimulation. Visual acuity was assessed using the Snellen test while somatosensory sensitivity was determined using vibration thresholds. The aims of the current study were to: (1) replicate a reliable MSI effect; (2) investigate the effect of unisensory functioning on VS RT facilitation; and (3) determine whether sensory functioning combination groups manifested differential MSI effects. Results revealed a significant VS RT facilitation effect that was influenced by somatosensory sensitivity but not visual acuity. That is, older adults with poor somatosensory sensitivity demonstrated significantly larger MSI effects than those with intact somatosensory sensitivity. Additionally, a significant interaction between stimulus condition and sensory functioning group suggested that the group with poor visual acuity and poor somatosensory functioning demonstrated the largest MSI effect compared to the other groups. In summary, the current study reveals that worse somatosensory functioning is associated with larger MSI effects in older adults. To our knowledge, this is first study to identify potential mechanisms behind increased RT facilitation in aging.
Keywords: Multisensory integration, visual acuity, somatosensory sensitivity, aging
1. Introduction
Every day we process concurrent sensory information received from both external and internal sources. In order to perceive the world as a coherent whole, as well as to make sense of our internal experiences, our sensory systems must interact seamlessly with each other (Talsma et al., 2010). Research investigating multisensory processing has been conducted for over a century (Calvert and Thesen, 2004; Todd, 1912), and the field has been expanding rapidly over the past several decades. Studies examining animals (Meredith and Stein, 1986; Wallace et al., 1996) and young adults (Foxe et al., 2000; Molholm et al., 2002; Teder-Salejarvi et al., 2002) have consistently demonstrated psychophysical and electrophysiological evidence for multisensory integration processes. Such processes are considered beneficial as they afford facilitation of reaction times (RTs), which allow for accurate and timely detection of stimulation (Calvert et al., 2004; Stein and Meredith, 1990).
Multisensory integration (MSI) effects have been demonstrated in older adults, though research with this population is scarce and most investigations have been limited to examining auditory-visual (AV) inputs (see Diederich et al., 2008; Hugenschmidt et al., 2009; Laurienti et al., 2006; Peiffer et al., 2007; Stephen et al., 2010). Findings from Laurienti et al. (2006) revealed decreased RTs in multisensory compared to unisensory conditions when analyzing speed of discrimination responses across auditory, visual, and AV inputs in young and old adults. Results indicated that compared to young adults, older adults exhibited significantly more multisensory RT facilitation. Using a simple reaction time task to investigate AV integration in both young and old adults, Peiffer et al. (2007) reported significantly faster RTs to multisensory compared to unisensory conditions, regardless of age group; however, the magnitude of the RT facilitation was significantly greater for the older adults. Using an AV focused attention task measuring saccadic RTs to unisensory and multisensory conditions, Diederich et al. (2008) also demonstrated greater multisensory enhancements in old compared to young adults. Similarly, on a cued discrimination task involving auditory and visual stimulation, older adults demonstrated significantly greater RT facilitation to multisensory trials compared to young adults (Hugenschmidt et al., 2009).
To determine which multisensory pairing resulted in the greatest RT facilitation for older adults, Mahoney et al. (2011) presented older and younger adults with unisensory (auditory, visual, and somatosensory) and multisensory combinations (AV, audio-somatosensory [AS], or visual-somatosensory [VS]). Results revealed that relative to young adults, older adults demonstrated the greatest RT facilitation to VS stimuli. Mahoney and colleagues have since reported that not all older adults demonstrate the same magnitude of VS integration. In fact, recent findings have indicated that older adults with larger RT facilitation effects manifest worse balance and increased falls (Mahoney et al., 2014), and maintain lower engagement in physical activities (Mahoney et al., 2015). Interestingly, other researchers have demonstrated that older adults exhibit difficulty attending to tactile stimulation in the presence of simultaneous visual distractors (Poliakoff et al., 2006a). Additionally, in a task of cross-modal temporal perception, older adults required presentation of a somatosensory stimulus prior to visual stimulus for spatially aligned inputs — a finding that was not observed in young adults (Poliakoff et al., 2006b). Collectively, these results have demonstrated: (a) a pattern of significantly increased behavioral RT facilitation to multisensory compared to unisensory stimulation in old relative to young adults; (b) allude to the importance of somatosensory functioning in aging; and (c) demonstrate that the VS combination is especially sensitive to age-related differences.
To date, however, the mechanism behind the increased RT facilitation in aging still remains unclear; however, several potential explanations have been proposed (see Freiherr et al., 2013; Laurienti and Hugenschmidt, 2012; Mozolic et al., 2012). The first potential explanation for increased RT facilitation in old relative to young adults relates to age-related cognitive slowing, or the reduction in speed of information processing. This explanation is unlikely here given the fact that older adults are capable of responding to unisensory visual and auditory inputs at similar speeds as young adults in very simple reaction time tasks which do not require significant cognitive effort (Peiffer et al., 2007; Yordanova et al., 2004). A second potential explanation for differential RT facilitation effects in aging relates to the fact that older adults manifest a time-window-of-integration (TWIN) that is nearly two times as wide as the young adults’ TWIN — which essentially increases the capacity to generate multisensory enhancements nearly two-fold (see Laurienti et al., 2006). However, according to Diederich and colleagues (2008), given that older adults’ responses are longer and more variable, the probability that multisensory interactions will occur during this wider TWIN is actually decreased, and therefore a wider TWIN cannot solely explain why older adults manifest larger RT facilitation effects (see also Mozolic et al., 2012).
A third potential explanation, which is most relevant to the current study, suggests that differences in age-related MSI effects could be related to known functional declines in sensory systems, whereby reduced functioning in individual sensory modalities could be directly related to increased MSI effects in aging (Freiherr et al., 2013; Laurienti and Hugenschmidt, 2012; Mozolic et al., 2012). Age-related decline has been demonstrated in various sensory systems (Da Silva et al., 2014; Hong et al., 2013; Owsley, 2011), including somatosensory functioning (Lin et al., 2005; Shaffer and Harrison, 2007), which has been linked to age-related alterations in speed of conduction (Bouche et al., 1993; Shaffer and Harrison, 2007; Taylor, 1984) and decreased innerva tion of the skin (Chang et al., 2004). In fact, vibration sensitivity appears to sustain the most significant decline with aging and is hypothesized to be an important indication of the aging process (Deshpande et al., 2008). Overall, declines in somatosensory functioning have been shown with increasing age (Kaye et al., 1994) and have been linked to functional deterioration in aging including increased risk for falls (Lord et al., 1994). In terms of visual processing, impairments have also been shown to increase with age (Baltes and Lindenberger, 1997; Kalina, 1997; Kaye et al., 1994; Spear, 1993), which in turn negatively affect older adults. Visual impairments have been linked to increased falls (Camicioli et al., 1997; Judge et al., 1995; Lord et al., 1994, 1999), difficulties with mobility (Owsley, 2011; Owsley and McGwin, 2004), and loss of independence and functional declines (LaForge et al., 1992).
While it has been questioned whether decreased functioning of individual sensory systems affect the mechanisms of MSI in older adults (Laurienti and Hugenschmidt, 2012; Mozolic et al., 2012), to our knowledge this particular research question has not been thoroughly evaluated in the context of aging. It does however seem logical to surmise that age-related sensory impairments would directly affect multisensory integrative processes (see also Laurienti and Hugenschmidt, 2012). In support, young adults with induced myopia demonstrated larger MSI effects compared to those with normal vision (Hairston et al., 2003). Additionally, the inverse effectiveness principle set forth by Stein and Meredith (1993) posits that the magnitude of MSI increases as the detectability of the unisensory stimuli decreases. Given that the detectability of unisensory stimuli likely decreases with chronological age, it seems reasonable to predict that this would be related to increased RT facilitation effects in aging.
To date several investigations in older adults have been conducted where inverse effectiveness was examined by specifically manipulating the unisensory inputs of AV combinations (Cienkowski and Carney, 2002; Tye-Murray et al., 2010). However, the idea of inverse effectiveness could technically be manipulated on either the delivering (physical stimulus input) or receiving (acuity/sensitivity of sensory system) end. Given that we know that the largest MSI effects occur when employing VS stimulation, we argue that the effect of sensory functioning on VS integration in aging can easily be assessed through the use of a simple reaction time task that does not expressly manipulate magnitude of sensory stimulation, but rather utilizes the individual’s degree of sensory functioning for both unisensory systems.
The current study was designed to accomplish the following three related aims: (1) to replicate a reliable VS RT facilitation effect in a large sample of non-demented older adults using a conventional test of the race model, which is considered the gold standard in the field; (2) to separately investigate the effect of visual and somatosensory functioning on VS RT facil itation effects in aging; and (3) to determine whether sensory functioning combination groups (poor and intact) that are based on clinically meaningful cuts would result in differential RT facilitation effects. We hypothesized that older adults with both poor visual (V) and somatosensory (S) functioning (Group 4: Low V/Low S functioning) would demonstrate significantly larger RT facilitation effects compared to the other sensory functioning combinations group, which include (Group 1: High V/High S; Group 2: Low V/High S; and Group 3: High V/Low S; see also Methods Section 2.5 for details). This study is novel in that it employs a three-prong approach to determine whether documented larger RT facilitation effects in aging are explained by age-related declines in sensory processing.
2. Material and Methods
2.1. Participants
Two hundred and fifty two older adults recruited from the Central Control of Mobility in Aging (CCMA) study at the Albert Einstein College of Medicine in Bronx, NY, USA, participated in this simple reaction time study. CCMA study procedures have been previously described (Holtzer et al., 2014a, b; Mahoney et al., 2014). Of the 194 individuals that successfully completed both the multisensory protocol and the sensory screening exam described below, 38 participants were excluded due to poor performance (two participants for significantly long and variable responses which raised the question of whether they fully understood the task and 36 participants for inadequate response accuracy [<70% on any condition]). Therefore, the current study consisted of a total of 156 participants. All study participants provided written informed consent to the experimental procedures, which were approved by the institutional review board.
2.2. Cognitive and Disease Status
Participants were deemed non-demented using reliable cut scores from the AD8 Dementia Screening Interview with cutoff score [greaterorequalslant] 2 (Galvin et al., 2005) and the Memory Impairment Screen (MIS; cutoff score < 5) (Buschke et al., 1999); diagnosis was subsequently confirmed using consensus clinical case conference procedures (Holtzer et al., 2008). Global cognitive status was assessed with the Repeatable Battery for Assessment of Neuropsychological Status (RBANS; Duff et al., 2008; Holtzer et al., 2014a) as part of the parent study and is provided here merely for characterization of the sample. Global disease summary scores (range 0–10) were obtained from dichotomous rating (presence or absence) of physician diagnosed diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson’s disease, chronic obstructive pulmonary disease, angina, and myocardial infarction (Mahoney et al., 2011, 2014). Additionally, all study participants received a comprehensive neurological exam as part of the parent study; presence of neuropathy (apparent in 5% of the total sample) was determined by the study physician and was adjusted for in our statistical analyses where appropriate.
2.3. Sensory Screening Exam
As part of the parent study, all participants were required to successfully complete a sensory screening exam, where visual, auditory, and somatosensory functioning were formally tested to ensure appropriateness for the study. Visual acuity was assessed using the Snellen eye chart. To be eligible for the CCMA study individuals had to be able to demonstrate binocular acuity of at least 20/100; however, for the purposes of the current study, participants with monocular blindness and/or visual acuity worse than 20/100 in either eye were not invited to participate. Each eye is tested individually and the best acuity, regardless of eye, is recorded. Participants wore their own glasses during the vision test. As part of the parent study and in an effort to ensure that participants who entered the study did not suffer from sensory deficits which could potentially influence their performance, hearing was also screened. A computerized tone-emitting otoscope that delivered lateral 20, 25, and 40 dB tones at 500, 1000, 2000, and 4000 Hz using E-prime 2.0 software (PST Psychology Software Tools, Inc., Pittsburgh, PA, USA); individuals were required to be able to hear a 2000 Hz tone at 40 dB.
Somatosensory sensitivity was assessed using quantitative sensory testing (QST), via the Vibratron II manufactured by Physitemp Instruments Inc.; this instrument is used for research procedures to assess peripheral sensory function (Gerr and Letz, 1988). The objective of this screening test is to determine the individuals’ lowest sensory threshold, measured in vibration units, for the upper extremities using a method of descending limits. The Vibratron is comprised of two vibrating posts and an examiner control box where constant vibrations are delivered at a frequency of 120 Hz to either post in a randomized order. The amplitude of vibration is measured in microns and is variably delivered by the tester. Each participant receives a standardized forced-choice protocol conducted by the study physician where they are asked to place the pad of their index finger on the posts and determine which post is actually vibrating. If the participant is correct, the amplitude of the vibration is reduced by ~10% for the next trial, continuing in a descending fashion until the first error is made. After the first error, the amplitude of vibration is increased to the amplitude of the last correct response and the trial is repeated. Testing is considered complete when the participant makes a total of two errors and the lowest accurate threshold is then recorded. Each index finger is tested individually and the lowest (i.e., best) sensory threshold regardless of finger is recorded.
2.4. Stimuli and Task Procedures
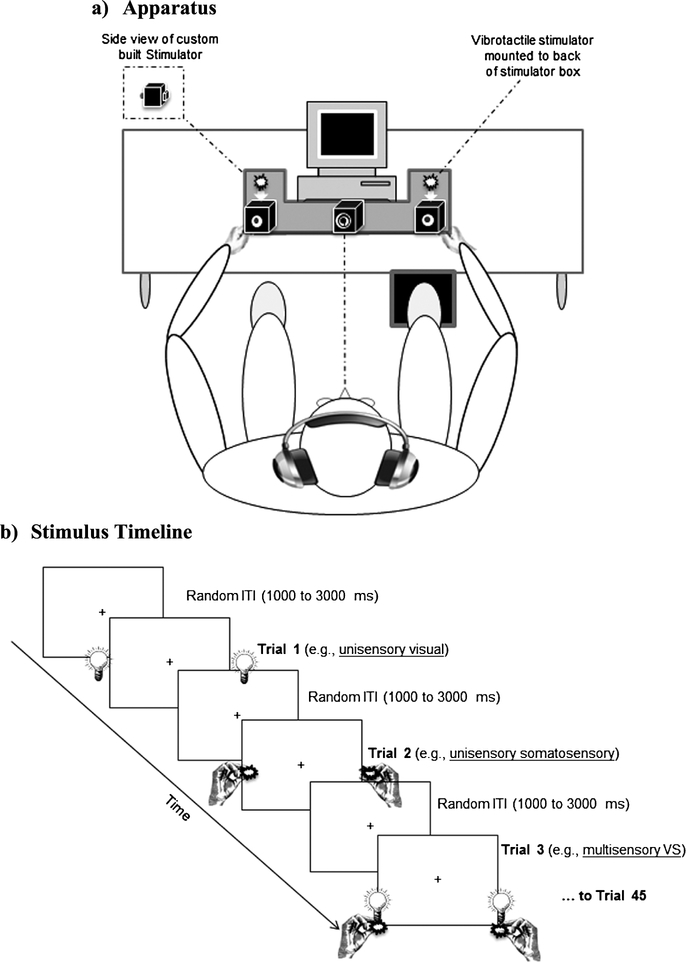
All participants completed a simple reaction time paradigm employing three sensory conditions: two unisensory (visual and somatosensory) and one multisensory (simultaneous visual-somatosensory). Visual and somatosensory stimuli were delivered through a custom built stimulus generator (Zenometrics, LLC, Peekskill, NY, USA) that consisted of two control boxes, each housing a 15.88 cm diameter blue light emitting diodes (LEDs) and a 30.48 mm × 20.32 mm × 12.70 mm plastic housing containing a vibrator motor with × 0.8G vibration amplitude (Fig. 1; see also Mahoney et al., 2015). As in our previous studies, bilateral stimulation for both visual and somatosensory stimulation was presented (Mahoney et al., 2011, 2014, 2015). The devices were connected to a network control center, which allowed direct control for each device through the testing computer’s parallel port. The devices were cycled on and off at precise predetermined intervals in any combination. A TTL (transistor–transistor-logic, 5 V, duration 100 ms) pulse was used to trigger the visual and somatosensory stimuli through E-Prime 2.0 software.
Figure 1.
Experimental procedures. (a) Apparatus: Participants rested hands comfortably on a custom-built apparatus while maintaining fixation on a target, and were required to make speeded responses to all stimuli, regardless of sensory modality, by pressing a foot pedal located under their right foot. (b) Sequence of events: Three blocks of V, S, and multisensory VS stimuli (45 trials per block) were randomly presented with random inter-trial-intervals (ITIs) of 1–3 s. From Mahoney et al. (2015).
The control boxes were mounted to an experimental apparatus, which participants comfortably rested their hands upon, with their index fingers strategically placed over the vibratory motors on the back of the box and their thumb on the front of the box, under the LED. A third dummy control box was placed in the center of the actual control boxes, at an equidistant length (28 cm). To ensure that the somatosensory stimuli were inaudible, each participant was provided with headphones over which continuous white noise was played at 60 dB SPL.
Participants were seated comfortably in a well-lit room and were required to look at a fixation point (a bull’s eye sticker with a central circle of 0.4 cm diameter placed on the dummy control box). The viewing distance of the observer from the fixation point was set at 57 cm. The fixation circle subtended a visual angle of 0.4 degrees. The three stimulus conditions were presented randomly with equal frequency over three blocks of 45 trials yielding a total of 135 trials. Each block was separated by a 20-s break in order to reduce fatigue and facilitate concentration. The next block started immediately after 20 s had elapsed. To prevent anticipatory effects, the inter-stimulus interval varied randomly from 1–3 s. The entire experiment took approximately seven minutes. Participants were instructed to respond to all stimuli by pressing a pedal located under their right foot as quickly as possible for each stimulus event.
2.5. Statistical Approach
Descriptive statistics, including means, standard deviations, and range were used to characterize the sample. For the RT data, RTs shorter than 100 ms and less than or greater than two standard deviations from the individual mean were treated as outliers. As previously described (Mahoney et al., 2015), participants were required to maintain response accuracy equal to or better than 70% across all stimulus conditions in order to be included in the analysis. Although this accuracy cut is arbitrary, it was chosen to allow for more individual variability in RTs due to potentially poor visual and somatosensory functioning. Individual mean RTs were subsequently calculated.
2.5.1. Test of the Race Model
In order to demonstrate a reliable VS RT facilitation effect, we employed a conventional test of the race model (see Colonius and Diederich, 2006). RTs were sorted in ascending order by stimulus condition and then averaged on an individual basis. For each participant, the RT range within the valid RTs was calculated across the three stimulus conditions and quantized into twenty bins from the fastest RT (or zero percentile) to the slowest RT (hundredth percentile) in 5% increments (0%, 5%, …, 95%, 100%). Differences between actual CP distributions [P(RTXY ≤ t)] and predicted CP distributions {min[P(RTX ≤ t)+P(RTY ≤ t)]} were calculated across each time bin across all participants (see Mahoney et al., 2014 for details); where values greater than zero are indicative of race model violation, providing support for multi-sensory integration processes. Given our specific aims, a test of the race model was first conducted for the entire sample (i.e., overall group n 156) and then conducted again for the four sensory functioning combination=groups (where each individual is only represented in one of the four groups).
2.5.2. Linear Mixed Effect Models
Separate Linear Mixed Effect Models (LMEMs) were employed to assess the effect of sensory functioning on VS RT facilitation, adjusted for age, gender, global disease status, and neuropathy (where appropriate). The models consisted of a three-level stimulus condition (visual (V), somatosensory (S), and visual-somatosensory (VS)), as well as visual acuity (first model) and somatosensory sensitivity (second model). To be consistent with established practices in visual acuity assessment literature (Falkenstein et al., 2008; Kaiser, 2009), raw Snellen scores were converted into logarithm of minimal angle of resolution (logMAR) scores. Lower logMAR scores are indicative of better visual acuity (e.g., 20/20 = 0 logMAR, 20/100 0.7 logMAR). Additionally, this conversion allows for the use of visual acuity as a continuous variable (Falkenstein et al., 2008; Kaiser, 2009). Raw somatosensory sensitivity scores were entered as a continuous measure (Pambianco et al., 2011). In accordance with accepted procedures (Falkenstein et al., 2008; Hong et al., 2013; Kaiser, 2009; Ong et al., 2012), the highest sensory functioning value was used for each analysis.
The advantage of the linear mixed effects models is that the heterogeneity and correlation of repeated measures under different conditions are taken into account (Laird and Ware, 1982). As part of our second aim, we first ran separate models to test the effect of visual acuity and somatosensory sensitivity on VS RT facilitation. The models included here take into account all RTs from all conditions. The first comparison in the model is the main effect of stimulus condition where RTs of the multisensory VS condition are compared to RTs of the somatosensory alone condition and then to RTs of the visual alone condition. Next, the main effect of visual acuity (model 1) or somatosensory sensitivity (model 2) is tested. Third, the interaction between condition and sensory functioning is assessed, relative to the two unisensory vs. multisensory VS comparisons. Lastly, the significance of the covariates is provided.
Given our third study aim of determining whether sensory functioning combination groups based on clinically meaningful cuts would manifest differential RT facilitation effects, a third LMEM with four groups was conducted. Individuals were divided into four sensory functioning combination groups: (1) individuals with high visual acuity and high somatosensory sensitivity (Group 1, High V/High S); (2) individuals with low visual acuity and high somatosensory sensitivity (Group 2, Low V/High S); (3) individuals with high visual acuity and low somatosensory sensitivity (Group 3, High V/Low S); and (4) individuals with low visual acuity and low somatosensory sensitivity (Group 4, Low V/Low S; reference group) based on data distribution and clinical indexing. In terms of visual acuity, individuals with visual acuity of 20/40 and better on the Snellen test (up to and including 0.3 logmar) were assigned into the high visual acuity group and individuals with visual acuity of 20/50 and worse (0.4 logMAR and higher) were assigned into the low visual acuity group. This cut is empirical, but it is however, in accordance with the International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM; Kobayashi, 2010) and the International Council of Ophthalmology (Ophthalmology, 2002) where scores on the Snellen test between 20/32 (logMAR = 0.2) and 20/63 (logMAR = 0.5) are indicative of mild vision loss. In terms of somatosensory sensitivity, individuals with a score of 1.3 and lower were assigned to the high sensitivity group and individuals with scores above 1.3 were assigned to the low sensitivity group. The 1.3 cut score employed here is indicative of the median value for the current sample.
The third LMEM is conducted in the same manner as the first and second LMEM, but instead of employing the continuous value for visual or somatosensory functioning, a four level sensory functioning combination group (defined above) with the reference group set to group 4 (the group of interest) is employed. While the reference group can be changed in the LMEMs, we make no specific hypotheses about Group 3 vs. 1, Group 3 vs. 2, and Group 2 vs. 1. Nonetheless, we provide these comparisons as they may provide the reader with additional information. Note that all data analyses were run using IBM’s Statistical Package for the Social Sciences (SPSS), Version 20.0 (IBM Corp., Released 2011).
3. Results
3.1. Demographics
One hundred and fifty six older adults (mean age 76.96 ± 6.07 years; 55% female) participated in the current experiment. None of the participants met criteria for dementia using established clinical consensus case-conference procedures (Holtzer et al., 2008). All participants were deemed relatively healthy as determined by their global health status. Table 1 delineates demographic information including but not limited to mean education level (in years), ethnicity, RBANS total score, visual acuity, somatosensory threshold, presence of neuropathy, and RTs in milliseconds (ms) overall and for the various experimental conditions. Demographic information is also provided for each of the four sensory functioning combination groups.
Table 1.
Participant demographics. Mean values (± SD) unless otherwise noted
| Overall | Group 1 High V/High S | Group 2 Low V/High S | Group 3 High V/Low S | Group 4 Low V/Low S | |
|---|---|---|---|---|---|
| Sample size | 156 | 62 | 21 | 53 | 20 |
| Age (years) | 76.96 (6.07) |
76.63 (6.03) |
76.33 (5.31) |
76.85 (6.05) |
78.90 (7.01) |
| Education (years) | 14.85 (2.84) |
15.11 (3.11) |
14.00 (2.21) |
14.96 (2.84) |
14.60 (2.56) |
| Global health scale score (0–10) |
1.25 (0.93) |
1.26 (0.97) |
1.24 (0.83) |
1.19 (0.94) |
1.40 (0.94) |
| % Female | 55 | 52 | 81 | 49 | 55 |
| % Caucasian | 80 | 82 | 62 | 77 | 95 |
| % with neuropathy (n) | 5 (7) |
5 (3) |
10 (2) |
2 (1) |
5 (1) |
| RBANS total score (standard score: 65–132) |
96.45 (12.22) |
100.00 (11.01) |
89.29 (12.50) |
97.02 (11.56) |
91.45 (13.21) |
| Visual acuity (logMAR, [−0.1–0.7]) |
0.26 (0.14) |
0.21 (0.09) |
0.44 (0.07) |
0.19 (0.10) |
0.43 (0.05) |
| Somatosensory sensitivity (vibration units, [0.1–4.3]) |
1.36 (0.82) |
0.76 (0.38) |
0.77 (0.46) |
2.05 (0.65) |
1.98 (0.60) |
| Overall RT (ms) | 361.74 (73.93) |
356.19 (71.56) |
342.28 (74.75) |
367.37 (66.39) |
384.52 (95.10) |
| RTs to somatosensory alone condition (ms) | 399.62 (84.22) |
392.07 (76.32) |
368.83 (84.25) |
406.90 (72.96) |
436.08 (119.76) |
| RTs to visual alone condition (ms) | 358.52 (73.53) |
351.66 (69.92) |
347.80 (80.55) |
364.47 (67.91) |
375.28 (91.04) |
| RTs to multisensory VS condition (ms) | 327.72 (72.40) |
325.39 (75.42) |
311.04 (69.41) |
331.08 (66.62) |
343.51 (81.62) |
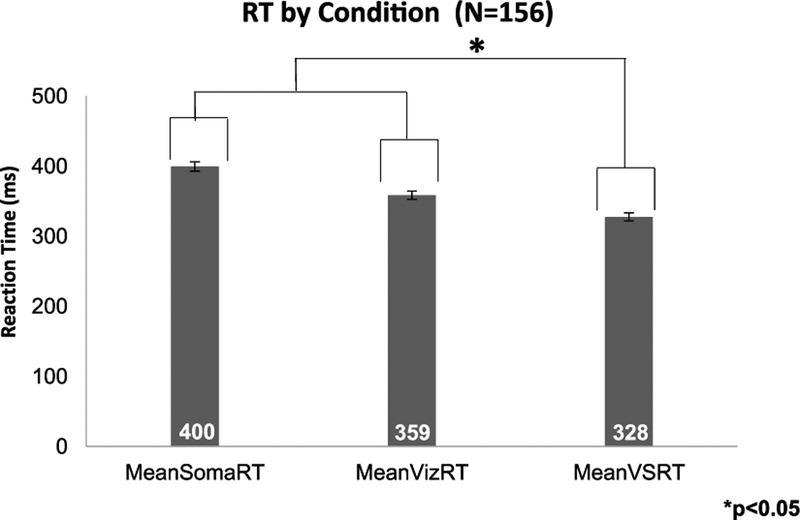
3.2. MSI Effect
As expected, RTs to multisensory trials were significantly shorter compared to the unisensory trials in the overall sample (see also Fig. 2). Results from the linear mixed effect models (LMEM), adjusted for age, gender, and global disease status revealed a significant multisensory RT facilitation effect (Table 2; p < 0.001).
Figure 2.
Averaged RT data by modality. Mean RT values (with SEM bars) for S, V, and VS conditions for all participants (n = 156). Averaged RTs to both unisensory constituents were significantly longer than RTs to the multisensory VS condition.
Table 2.
Linear mixed effects model results for: (1) Multisensory RT facilitation; (2) effect of visual acuity; (3) visual acuity by multisensory RT facilitation; and (4) adjustments
| Group/Adjustment | Condition | Estimate | 95% confidence interval | SE | p value | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| (1) | RT facilitation | Multisensory VS vs. S | 70.59 | 58.45 | 82.73 | 6.17 | <0.001 |
| Multisensory VS vs. V | 24.47 | 12.33 | 36.61 | 6.17 | <0.001 | ||
| (2) | Visual acuity | logMAR | −11.33 | −101.07 | 78.40 | 45.47 | 0.803 |
| (3) | Visual acuity × RT facilitation | logMAR × [VS vs. S] | 5.05 | −36.39 | 46.49 | 21.06 | 0.811 |
| logMAR × [VS vs. V] | 24.39 | −17.05 | 65.83 | 21.06 | 0.248 | ||
| (4) | Adjustments | Age | −0.57 | −2.50 | 1.36 | 0.98 | 0.561 |
| Gender | −31.19 | −54.75 | −7.64 | 11.92 | 0.010 | ||
| GHS | 0.72 | −11.79 | 13.22 | 6.33 | 0.910 | ||
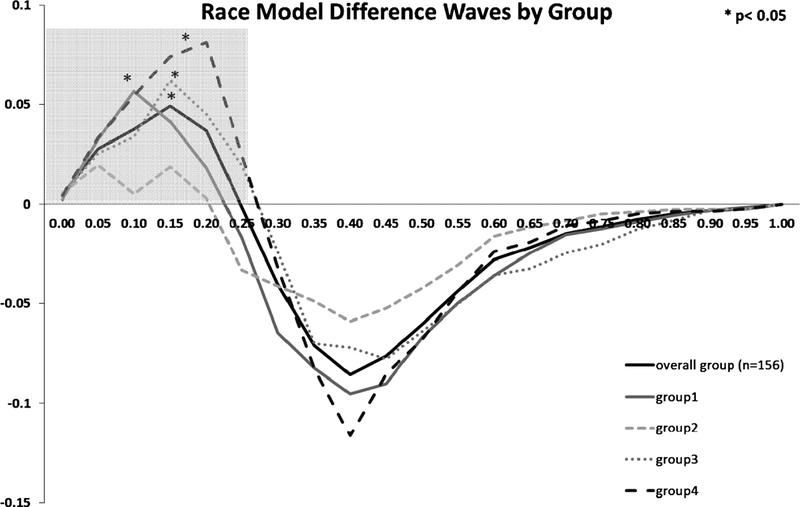
3.3. Race Model Results
Difference waveforms between actual and predicted CP distributions for the overall group (solid black trace), as well as for the four sensory functioning combination groups are plotted in Fig. 3. Again, positive values represent a violation in the race model (i.e., support for multisensory integrative processing) and were significant at the p < 0.05 level over the fastest quartile (0.0 to 0.25) of RTs (shaded box) for the overall sample. Thus, we demonstrate a reliable VS RT facilitation effect in this large sample of older adults. Further, the difference waveforms for Group 4 (Low V/Low S; dashed black trace) revealed the largest amount of VS RT facilitation; a finding that is directly in line with our hypothesis. Group 1 (High V/High S; solid grey trace) and Group 3 (High V/Low S; dotted grey trace) demonstrated similar significant RT facilitations, peaking at slightly different probability bins. Noteworthy, the difference wave for Group 2 (Low V/High S; dashed grey trace) violated the race model, however revealed the smallest amount of RT facilitation. Such a finding could be directly related to the fact that Group 2 demonstrated the fastest unisensory RTs, thereby masking the overall VS facilitation effect.
Figure 3.
Test of the Race Model. The cumulative probability difference waves (actual minus predicted) over the trajectory of averaged responses for the overall group and the four sensory combination groups. The shaded grey box represents the fastest quartile of RTs (i.e., 25th percentile). Values greater than zero indicate violations of the race model.
In order to determine the reliability of each violation, one-way ANOVAs were conducted over the first quartile (i.e., 25 %) of all RTs. Significant race model violation was obtained for the overall group as well as for all group combinations, except Group 2 (Low V/High S; see asterisks on Fig. 3).
3.4. MSI and Unisensory Functioning
In the first LMEM, the interaction between stimulus condition and visual acuity was not significant, F (2, 308) = 0.748, p = 0.474. This finding indicates that visual acuity as measured by the Snellen test did not moderate the RT facilitation effect (i.e., difference between RTs to VS compared to V and S conditions; see Table 2).
In the second LMEM, there was a significant interaction between stimulus condition and somatosensory sensitivity [F (2, 308) = 6.697, p = 0.001], revealing that somatosensory sensitivity moderated the RT facilitation effect between VS and S (B = 11.84, 95% CI [5.09, 18.58], p = 0.001), but not between VS and V (B = 2.32, 95% CI [−4.42, 9.07], p = 0.499; see Table 3) stimulus conditions. Collectively, these results suggest that somatosensory sensitivity but not visual acuity (measured by the Snellen) influenced VS RT facilitation.
Table 3.
Linear mixed effects model results for: (1) Multisensory RT facilitation; (2) effect of somatosensory sensitivity; (3) somatosensory sensitivity by multisensory RT facilitation; and (4) adjustments
| Group/Adjustment | Condition | Estimate | 95% confidence interval | SE | p value | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| (1) | RT facilitation | Multisensory VS vs. S | 55.85 | 45.16 | 66.53 | 5.43 | <0.001 |
| Multisensory VS vs. V | 27.66 | 16.97 | 38.34 | 5.43 | <0.001 | ||
| (2) | Somatosensory sensitivity | Vibration threshold | 1.44 | −13.42 | 16.30 | 7.53 | 0.848 |
| (3) | Somatosensory sensitivity × RT facilitation | Vibration threshold × [VS vs. S] | 11.84 | 5.09 | 18.58 | 3.43 | 0.001 |
| Vibration threshold × [VS vs. V] | 2.32 | −4.42 | 9.07 | 3.43 | 0.499 | ||
| (4) | Adjustments | Age | −0.71 | −2.65 | 1.24 | 0.98 | 0.473 |
| Gender | −31.10 | −54.68 | −7.53 | 11.93 | 0.010 | ||
| GHS | 1.09 | −11.51 | 13.69 | 6.38 | 0.864 | ||
| Neuropathy | −13.75 | −70.62 | 43.11 | 28.78 | 0.633 | ||
3.5. MSI and Sensory Functioning Combinations
Our hypothesis was that individuals with poor visual acuity and poor somatosensory sensitivity (Group 4: Low V/Low S) would demonstrate larger VS RT facilitation effects compared to elders in the other three sensory functioning combination groups. We examined the effect of combined visual acuity and somatosensory sensitivity on VS RT facilitation by running a third LMEM on VS RT facilitation with a four level group (Group 4 set as reference) and a three level RT facilitation type (multisensory VS condition set as reference; see also Table 4). Results demonstrated a significant interaction between stimulus condition and sensory functioning combination group, F (6, 304) = 3.083, p = 0.006, revealing that sensory functioning combination group moderated the difference between RTs to multisensory compared to unisensory conditions. Specifically, compared to the group with Low V/Low S (Group 4) functioning, participants with High V/High S (Group 1) and Low V/High S (Group 2) demonstrated significantly less RT facilitation effects for S compared to VS stimulus condition: Group 1, B 25.89, 95% CI [43.60,−8.19], p 0.004, and Group 2, B 34. = −95% CI [56.28, − p = −77, 13.26],=There was no difference between the Low V/Low−S (Group= 0.002.− 4) andthe High V/Low S (Group 3), although there was a trend toward significance, B = −16.75, 95% CI [−34.82, 1.32], p 0.longer =069. Taken together, these data suggest that RT facilitation effects are associated with impairments in somatosensory sensitivity. Additional group comparisons are reported in Table 5, where the comparisons of interest are intentionally reiterated. The results of the RT facilitation effects for V compared to VS stimulus condition were not significant for any sensory functioning combination group.
Table 4.
Linear mixed effects model results for: (1) Multisensory RT facilitation; (2) effect of group status; (3) group status by multisensory RT facilitation; and (4) adjustments
| Group/Adjustment | Condition | Estimate | 95% confidence interval | SE | p value | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| (1) | RT facilitation | Multisensory VS vs. S | 92.56 | 77.17 | 107.96 | 7.82 | < 0.001 |
| Multisensory VS vs. V | 31.76 | 16.37 | 47.16 | 7.82 | < 0.001 | ||
| (2) | Group status | Group 4 vs. Group 1 | −20.89 | −59.69 | 17.91 | 19.66 | 0.289 |
| Group 4 vs. Group 2 | −26.16 | −73.81 | 21.49 | 24.14 | 0.280 | ||
| Group 4 vs. Group 3 | −16.05 | −55.69 | 23.59 | 20.08 | 0.425 | ||
| (3) | Group status × RT facilitation | [Group 4 vs. Group 1] × [Multisensory VS vs. S] | −25.89 | −43.59 | −8.19 | 9.00 | 0.004 |
| [Group 4 vs. Group 2] × [Multisensory VS vs. S] | −34.77 | −56.28 | −13.26 | 10.93 | 0.002 | ||
| [Group 4 vs. Group 3] × [Multisensory VS vs. S] | −16.75 | −34.82 | 1.32 | 9.18 | 0.069 | ||
| [Group 4 vs. Group 1] × [Multisensory VS vs. V] | −5.50 | −23.20 | 12.21 | 9.00 | 0.542 | ||
| [Group 4 vs. Group 2] × [Multisensory VS vs. V] | 5.00 | −16.51 | 26.51 | 10.93 | 0.648 | ||
| [Group 4 vs. Group 3] × [Multisensory VS vs. V] | 1.62 | −16.44 | 19.69 | 9.18 | 0.860 | ||
| (4) | Adjustments | Age | −0.80 | −2.74 | 1.13 | 0.98 | 0.414 |
| Gender | −30.12 | −54.18 | −6.05 | 12.18 | 0.015 | ||
| GHS | 0.61 | −11.98 | 13.21 | 6.37 | 0.924 | ||
| Neuropathy | −10.15 | −67.44 | 47.13 | 28.99 | 0.727 | ||
Table 5.
Group status × RT facilitation [Multisensory VS vs. S] results for all group combinations*
| Condition | Estimate | 95% confidence interval | SE | p value | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| [Group 4 vs. Group 1] × [Multisensory VS vs. S] | −25.89 | −43.59 | −8.19 | 9.00 | 0.004 | Planned comparisons | |
| [Group 4 vs. Group 2] × [Multisensory VS vs. S] | −34.77 | −56.28 | −13.26 | 10.93 | 0.002 | ||
| [Group 4 vs. Group 3] × [Multisensory VS vs. S] | −16.75 | −34.82 | 1.32 | 9.18 | 0.069 | ||
| [Group 3 vs. Group 1] × [Multisensory VS vs. S] | −9.14 | −22.02 | 3.74 | 6.54 | 0.164 | ||
| [Group 3 vs. Group 2] × [Multisensory VS vs. S] | −18.02 | −35.77 | −0.27 | 9.02 | 0.047 | ||
| [Group 2 vs. Group 1] × [Multisensory VS vs. S] | 8.88 | −8.50 | 26.26 | 8.83 | 0.315 | ||
The first three comparisons are intentionally replicated from Table 4; however in order to provide results for all group comparisons, the same LMEM was run two additional times with a different reference. Across all three LMEMs, regardless of reference group, the interaction of group with RT facilitation (multisensory VS vs. V) was never significant — these data are not presented.
4. Discussion
In the current study we demonstrate a reliable VS RT facilitation effect and further reveal a significant effect of somatosensory functioning on VS RT facilitation effects in aging. Visual acuity as measured by the Snellen test did not have a significant effect on VS RT facilitation alone. However, the combined effect of visual acuity and somatosensory sensitivity on VS RT facilitation was significant. Specifically, compared to the sensory function combination group with low vision and low somatosensory sensitivity, the groups with high somatosensory sensitivity demonstrated significantly less (i.e., more efficient) RT facilitation effects for S compared to VS stimulus conditions.
Findings from this study are in line with our hypothesis and clearly demonstrate that individuals with both poor somatosensory and poor visual functioning demonstrate the greatest multisensory RT facilitation effect. This discovery is congruent with previous studies that demonstrate that larger RT facilitation effects in aging were associated with increased falls, worse balance, and less engagement in physical activities (Mahoney et al., 2014, 2015). Here significant multisensory RT facilitation effects are once again reported (Laurienti et al., 2006; Mahoney et al., 2011, 2014; Peiffer et al., 2007), but of particular importance, older adults with worse somatosensory sensitivity appear to exhibit significantly larger VS multisensory RT facilitation effects compared to those with intact somatosensory functioning. Visual acuity, as measured by the Snellen eye chart, was not solely associated with VS RT facilitation in older adults in the current study. The novelty of this study is that findings suggest that deficits in somatosensory functioning in particular could potentially be one explanation for larger VS RT facilitation effects in older adults.
When assessing the visual acuity and somatosensory sensitivity combinations, a differential relationship was clearly demonstrated. Specifically the group with poor functioning in both sensory modalities (Group 4) demon strated the greatest VS RT facilitation effect for the VS compared to S stimuli (~92 ms). This finding is also apparent in the test of the race model, where individuals in Group 4 demonstrated the largest RT facilitation effect (difference in actual vs. predicted cumulative probability). In terms of the LMEM results, there was no difference in VS RT facilitation for the two groups with poor somatosensory functioning (Groups 3 and 4; p = 0.07) suggesting that both groups exhibited equally large VS RT facilitation effects. Additionally, there was no difference in RT facilitation effects for individuals with intact somatosensory functioning (Groups 1 and 2; p = 0.31). This finding alludes to the importance of somatosensory functioning for efficient multisensory processing in aging.
While not specifically tested, the inherent changes of the somatosensory cortex with age (Raz et al., 2004) could potentially explain the strong relationship between somatosensory function and VS integration reported here. Decline in vibration sense with age has been discussed as the result of loss of sensory receptors (Deshpande et al., 2008; Kenshalo, 1986; Shaffer and Harrison, 2007; Stuart et al., 2003; Verrillo, 1980). Findings from our study suggest that the reduced sensitivity of somatosensory processing, which could also potentially be caused by alterations in neural architecture of somatosensory cortex due to age-related declines, directly impacts the efficiency of VS integration in older adults.
Visual acuity did not have a significant effect on VS RT facilitation in the current study. While it could be argued that the Snellen is an imperfect measure (Hussain et al., 2006; Kaiser, 2009) that may not provide sensitive results to detect differences in multisensory processing, the Snellen is a widely used measure of visual acuity employed in many research and clinical settings, and even the department of motor vehicles (Baltes and Lindenberger, 1997; Falkenstein et al., 2008; Kaiser, 2009; Lindenberger and Ghisletta, 2009). Furthermore, visual acuity assessed by the Snellen measures acuity in central fovea; however, stimulation was presented off central fixation in the current experiment and thus could have captured different aspects of vision. Nevertheless, given that the visual stimuli used in the current experiment were very salient (bright blue LEDs) and the current sample of healthy older adults demonstrated fairly good visual acuity overall (~20/35), the differential performance among their RTs depending on stimulus condition was likely not affected by visual acuity. Interestingly, contrast sensitivity was found to be a more significant factor in the prediction of falls among older adults compared to visual acuity (Lord and Dayhew, 2001). Therefore, future studies may choose to assess whether contrast sensitivity plays a role in MSI when employing visual stimuli that are varied in contrast and provide more naturalistic stimulation than the salient LED lights employed in the current study.
To our knowledge, this study is the first to investigate the potential cause of increased RT facilitation in aging. While it appears that somatosensory sensitivity was more significantly associated with VS RT facilitation than visual acuity, we cannot ignore the fact that the older adults with both poor somatosensory sensitivity and poor visual acuity demonstrated the greatest RT facilitation effect. It is well known that both somatosensory functioning (Chang et al., 2004; Lin et al., 2005) and visual functioning (Spear, 1993) decline with age. Here we demonstrate, for the first time, that impairments in sensory functioning are associated with increased MSI effects in older adults. The clinical implications of this study point to the importance of monitoring somatosensory functioning in older adults. Additionally, given the findings of increased VS RT facilitation in individuals with poor sensory functioning, MSI training involving somatosensory stimulation could be potentially implemented to help older adults achieve more efficient integration of multisensory stimuli, as it has been achieved in both young (Powers et al., 2009) and older (Setti et al., 2014) adults. This is important, as efficient MSI has been linked to accurate and timely detection of stimulation, which could ultimately be important in life-threatening situations where every millisecond counts. Based on the current findings, it might be of interest to tailor thresholds of sensory stimulation to optimize MSI for each individual; however, additional studies are required to assess the viability of such considerations.
The current study is not without its limitations. The sample consisted of relatively healthy community dwelling older adults, and therefore, the results are somewhat limited to similar populations and cannot be extrapolated onto clinical populations with severe impairments in sensory functioning, persons with dementia, or residents of assisted living facilities. It would, however, be of interest to assess the effect of significantly impaired sensory functioning on VS RT facilitation. On that note, visual impairments were not terribly severe for most participants. This could potentially explain why visual acuity did not appear to moderate the VS RT facilitation effects, as it could have masked the potential to observe increased RT facilitation effects. Additionally, it is noteworthy that the procedures used to determine visual acuity (Snellen test) are very different compared to the visual stimuli employed in the current experiment (LED flashes). On the contrary, the procedures to determine somatosensory acuity (vibration threshold detection) were similar to the somatosensory stimuli employed in the current experiment, in that they both utilized vibratory stimulation. Future studies may want to employ similar visual stimuli, perhaps even contrast sensitivity stimuli discussed above, for tests of visual acuity and MSI. Lastly, as indicated above, visual stimuli in the SRT task were very salient and potentially more salient than the somatosensory stimuli. The intensity of the physical stimuli, which were strategically employed to measure RTs to more naturalistic stimuli that individuals are faced with daily (see Mahoney et al., 2015) likely resulted in significantly shorter RTs in visual alone conditions which could have impacted the current findings. Therefore, it might be useful for future experiments to calibrate the stimulus intensity of both the visual and somatosensory inputs.
Acknowledgements
Research was supported by funding from the National Institute on Aging (R01AG036921–01A1 and R01AG044007–01A1). Special thanks to the participants and CCMA research assistants for their assistance with data collection. Additionally, sincere gratitude to Constantin M. Trantzas of Zenometrics, LLC, for designing and implementing the custom built stimulus generator.
References
- Baltes PB and Lindenberger U (1997). Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol. Aging 12, 12–21. [DOI] [PubMed] [Google Scholar]
- Bouche P, Cattelin F, Saint-Jean O, Leger JM, Queslati S, Guez D, Moulonguet A, Brault Y, Aquino JP and Simunek P (1993). Clinical and electrophysiological study of the peripheral nervous system in the elderly, J. Neurol 240, 263–268. [DOI] [PubMed] [Google Scholar]
- Buschke H, Kuslansky G, Katz M, Stewart WF, Sliwinski MJ, Eckholdt HM and Lipton RB (1999). Screening for dementia with the memory impairment screen, Neurology 52, 231–238. [DOI] [PubMed] [Google Scholar]
- Calvert GA and Thesen T (2004). Multisensory integration: methodological approaches and emerging principles in the human brain, J. Physiol. Paris 98, 191–205. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Spence C and Stein BE (Eds) (2004). The Handbook of Multisensory Processes. MIT Press, Cambridge, MA, USA. [Google Scholar]
- Camicioli R, Panzer VP and Kaye J (1997). Balance in the healthy elderly: posturography and clinical assessment, Arch. Neurol 54, 976–981. [DOI] [PubMed] [Google Scholar]
- Chang YC, Lin WM and Hsieh ST (2004). Effects of aging on human skin innervation, Neuroreport 15, 149–153. [DOI] [PubMed] [Google Scholar]
- Cienkowski KM and Carney AE (2002). Auditory–visual speech perception and aging, Ear Hear. 23, 439–449. [DOI] [PubMed] [Google Scholar]
- Colonius H and Diederich A (2006). The race model inequality: interpreting a geometric measure of the amount of violation, Psychol. Rev 113, 148–154. [DOI] [PubMed] [Google Scholar]
- Da Silva LA, Lin SM, Teixeira MJ, De Siqueira JT, Jacob Filho W and De Siqueira S (2014). Sensorial differences according to sex and ages, Oral Dis 20, e103–e110. DOI: 10.1111/odi.12145 [DOI] [PubMed] [Google Scholar]
- Deshpande N, Metter EJ, Ling S, Conwit R and Ferrucci L (2008). Physiological correlates of age-related decline in vibrotactile sensitivity, Neurobiol. Aging 29, 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich A, Colonius H and Schomburg A (2008). Assessing age-related multisensory enhancement with the time-window-of-integration model, Neuropsychologia 46, 2556–2562. [DOI] [PubMed] [Google Scholar]
- Duff K, Humphreys Clark JD, O’bryant SE, Mold JW, Schiffer RB and Sutker PB (2008). Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers, Arch. Clin. Neuropsychol 23, 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein IA, Cochran DE, Azen SP, Dustin L, Tammewar AM, Kozak I and Freeman WR (2008). Comparison of visual acuity in macular degeneration patients measured with snellen and early treatment diabetic retinopathy study charts, Ophthalmology 115, 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Morocz IA, Murray MM, Higgins BA, Javitt DC and Schroeder CE (2000). Multisensory auditory–somatosensory interactions in early cortical processing revealed by high-density electrical mapping, Brain Res. Cogn. Brain Res 10, 77–83. [DOI] [PubMed] [Google Scholar]
- Freiherr J, Lundström JN, Habel U and Reetz K (2013). Multisensory integration mechanisms during aging, Front. Hum. Neurosci 7, 863 DOI: 10.3389/fnhum.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, Miller JP, Storandt M and Morris JC (2005). The AD8: a brief informant interview to detect dementia, Neurology 65, 559–564. [DOI] [PubMed] [Google Scholar]
- Gerr FE and Letz R (1988). Reliability of a widely used test of peripheral cutaneous vibration sensitivity and a comparison of two testing protocols, Br. J. Ind. Med 45, 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston WD, Laurienti PJ, Mishra G, Burdette JH and Wallace MT (2003). Multi-sensory enhancement of localization under conditions of induced myopia, Exp. Brain Res 152, 404–408. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, Hall CB and Lipton RB (2008). Within-person across-neuropsychological test variability and incident dementia, JAMA 300, 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney J and Verghese J (2014a). Intraindividual variability in executive functions but not speed of processing or conflict resolution predicts performance differences in gait speed in older adults, J. Gerontol. A Biol. Sci. Med. Sci 69, 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Wang C and Verghese J (2014b). Performance variance on walking while talking tasks: theory, findings, and clinical implications, Age (Dordr.) 36, 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T, Mitchell P, Rochtchina E, Fong CS, Chia EM and Wang JJ (2013). Long-term changes in visual acuity in an older population over a 15-year period: The Blue Mountains Eye Study, Ophthalmology 120, 2091–2099. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Mozolic JL and Laurienti PJ (2009). Suppression of multisensory integration by modality-specific attention in aging, Neuroreport 20, 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain B, Saleh GM, Sivaprasad S and Hammond CJ (2006). Changing from Snellen to LogMAR: debate or delay? Clin. Experiment Ophthalmol 34, 6–8. [DOI] [PubMed] [Google Scholar]
- IBM Corp. (Released 2011). SPSS Statistics for Windows, 20.0 edn. IBM Corp., Armonk, NY, USA. [Google Scholar]
- International Council of Ophthalmology (2002). Visual standards: aspects and ranges of vision loss with emphasis on population surveys [online] Sydney, Australia: Available at: http://www.icoph.org/downloads/visualstandardsreport.pdf. [Google Scholar]
- Judge JO, King MB, Whipple R, Clive J and Wolfson LI (1995). Dynamic balance in older persons: effects of reduced visual and proprioceptive input, J. Gerontol. A Biol. Sci. Med. Sci 50, M263–M270. [DOI] [PubMed] [Google Scholar]
- Kaiser PK (2009). Prospective evaluation of visual acuity assessment: a comparison of Snellen versus ETDRS charts in clinical practice (an AOS thesis), Trans. Am. Ophthalmol. Soc 107, 311–324. [PMC free article] [PubMed] [Google Scholar]
- Kalina RE (1997). Seeing into the future. Vision and aging, West J. Med 167, 253–257. [PMC free article] [PubMed] [Google Scholar]
- Kaye JA, Oken BS, Howieson DB, Howieson J, Holm LA and Dennison K (1994). Neurologic evaluation of the optimally healthy oldest old, Arch. Neurol 51, 1205–1211. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR Sr (1986). Somesthetic sensitivity in young and elderly humans, J. Gerontol 41, 732–742. [DOI] [PubMed] [Google Scholar]
- Kobayashi L (2010). ICD-9-CM Inpatient Coding Reference and Study Guide. Dog Ear Publishing, LLC, Indianapolis, IN, USA. [Google Scholar]
- Laforge RG, Spector WD and Sternberg J (1992). The relationship of vision and hearing impairment to one-year mortality and functional decline, J. Aging Health 4, 126–148. [Google Scholar]
- Laird NM and Ware JH (1982). Random-effects models for longitudinal data, Biometrics 38, 963–974. [PubMed] [Google Scholar]
- Laurienti PJ and Hugenschmidt CE (2012). Multisensory processes in old age, in: Multi-sensory Development, Bremner AJ, Lewkowicz DJ and Spence C (Eds), pp. 251–270. Oxford University Press, Oxford, UK. [Google Scholar]
- Laurienti PJ, Burdette JH, Maldjian JA and Wallace MT (2006). Enhanced multisensory integration in older adults, Neurobiol. Aging 27, 1155–1163. [DOI] [PubMed] [Google Scholar]
- Lin YH, Hsieh SC, Chao CC, Chang YC and Hsieh ST (2005). Influence of aging on thermal and vibratory thresholds of quantitative sensory testing, J. Peripher. Nerv. Syst 10, 269–281. [DOI] [PubMed] [Google Scholar]
- Lindenberger U and Ghisletta P (2009). Cognitive and sensory declines in old age: gauging the evidence for a common cause, Psychol. Aging 24, 1–16. [DOI] [PubMed] [Google Scholar]
- Lord SR and Dayhew J (2001). Visual risk factors for falls in older people, J. Am. Geriatr. Soc 49, 508–515. [DOI] [PubMed] [Google Scholar]
- Lord SR, Ward JA, Williams P and Anstey KJ (1994). Physiological factors associated with falls in older community-dwelling women, J. Am. Geriatr. Soc 42, 1110–1117. [DOI] [PubMed] [Google Scholar]
- Lord SR, Rogers MW, Howland A and Fitzpatrick R (1999). Lateral stability, sensori-motor function and falls in older people, J. Am. Geriatr. Soc 47, 1077–1081. [DOI] [PubMed] [Google Scholar]
- Mahoney JR, Li PC, Oh-Park M, Verghese J and Holtzer R (2011). Multisensory integration across the senses in young and old adults, Brain Res 1426, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JR, Holtzer R and Verghese J (2014). Visual-somatosensory integration and balance: evidence for psychophysical integrative differences in aging, Multisens. Res 27, 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JR, Dumas K and Holtzer R (2015). Visual-somatosensory integration is linked to physical activity level in older adults, Multisens. Res 28, 11–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA and Stein BE (1986). Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration, J. Neurophysiol 56, 640–662. [DOI] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Murray MM, Javitt DC, Schroeder CE and Foxe JJ (2002). Multisensory auditory–visual interactions during early sensory processing in humans: a high-density electrical mapping study, Brain Res. Cogn. Brain Res 14, 115–128. [DOI] [PubMed] [Google Scholar]
- Mozolic JL, Hugenschmidt CE, Peiffer AM and Laurienti PJ (2012). Multisensory integration and aging, in: The Neural Bases of Multisensory Processes, Murray MM and Wallace MT (Eds), pp. 381–392. CRC Press, Boca Raton, FL, USA. [PubMed] [Google Scholar]
- Ong SY, Cheung CY, Li X, Lamoureux EL, Ikram MK, Ding J, Cheng CY, Haaland BA, Saw SM, Venketasubramanian N, Chen CP and Wong TY (2012). Visual impairment, age-related eye diseases, and cognitive function: the Singapore Malay Eye study, Arch. Ophthalmol 130, 895–900. [DOI] [PubMed] [Google Scholar]
- Owsley C (2011). Aging and vision, Vis. Res 51, 1610–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C and McGwin G Jr (2004). Association between visual attention and mobility in older adults, J. Am. Geriatr. Soc 52, 1901–1906. [DOI] [PubMed] [Google Scholar]
- Pambianco G, Costacou T, Strotmeyer E and Orchard TJ (2011). The assessment of clinical distal symmetric polyneuropathy in type 1 diabetes: a comparison of methodologies from the Pittsburgh Epidemiology of Diabetes Complications Cohort, Diabetes Res. Clin. Pract 92, 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer AM, Mozolic JL, Hugenschmidt CE and Laurienti PJ (2007). Age-related multisensory enhancement in a simple audiovisual detection task, Neuroreport 18, 1077–1081. [DOI] [PubMed] [Google Scholar]
- Poliakoff E, Ashworth S, Lowe C and Spence C (2006a). Vision and touch in ageing: crossmodal selective attention and visuotactile spatial interactions, Neuropsychologia 44, 507–517. [DOI] [PubMed] [Google Scholar]
- Poliakoff E, Shore DI, Lowe C and Spence C (2006b). Visuotactile temporal order judgments in ageing, Neurosci. Lett 396, 207–211. [DOI] [PubMed] [Google Scholar]
- Powers AR, Hillock AR and Wallace MT (2009). Perceptual training narrows the temporal window of multisensory binding, J. Neurosci 29, 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A and Acker JD (2004). Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume, Neurobiol. Aging 25, 377–396. [DOI] [PubMed] [Google Scholar]
- Setti A, Stapleton J, Leahy D, Walsh C, Kenny RA and Newell FN (2014). Improving the efficiency of multisensory integration in older adults: audio–visual temporal discrimination training reduces susceptibility to the sound-induced flash illusion, Neuropsychologia 61, 259–268. [DOI] [PubMed] [Google Scholar]
- Shaffer SW and Harrison AL (2007). Aging of the somatosensory system: a translational perspective, Phys. Ther 87, 193–207. [DOI] [PubMed] [Google Scholar]
- Spear PD (1993). Neural bases of visual deficits during aging, Vis. Res 33, 2589–2609. [DOI] [PubMed] [Google Scholar]
- Stein BE and Meredith MA (1990). Multisensory integration. Neural and behavioral solutions for dealing with stimuli from different sensory modalities, Ann. N. Y. Acad. Sci 608, 51–65; discussion 65–70. [DOI] [PubMed] [Google Scholar]
- Stein BE and Meredith MA (1993). The Merging of the Senses. MIT Press, Cambridge, MA, USA. [Google Scholar]
- Stephen JM, Knoefel JE, Adair J, Hart B and Aine CJ (2010). Aging-related changes in auditory and visual integration measured with MEG, Neurosci. Lett 484, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart M, Turman AB, Shaw J, Walsh N and Nguyen V (2003). Effects of aging on vibration detection thresholds at various body regions, BMC Geriatr. 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsma D, Senkowski D, Soto-Faraco S and Woldorff MG (2010). The multifaceted interplay between attention and multisensory integration, Trends Cogn. Sci 14, 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PK (1984). Non-linear effects of age on nerve conduction in adults, J. Neurol. Sci 66, 223–234. [DOI] [PubMed] [Google Scholar]
- Teder-Salejarvi WA, Mcdonald JJ, Di Russo F and Hillyard SA (2002). An analysis of audio-visual crossmodal integration by means of event-related potential (ERP) recordings, Brain Res. Cogn. Brain Res 14, 106–114. [DOI] [PubMed] [Google Scholar]
- Todd JW (1912). Reaction to multiple stimuli, Arch. Psychol 3, 49–52. [Google Scholar]
- Tye-Murray N, Sommers M, Spehar B, Myerson J and Hale S (2010). Aging, audiovisual integration, and the principle of inverse effectiveness, Ear Hear. 31, 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrillo RT (1980). Age related changes in the sensitivity to vibration, J. Gerontol 35, 185–193. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Wilkinson LK and Stein BE (1996). Representation and integration of multiple sensory inputs in primate superior colliculus, J. Neurophysiol 76, 1246–1265. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Hohnsbein J and Falkenstein M (2004). Sensorimotor slowing with ageing is mediated by a functional dysregulation of motor-generation processes: evidence from high-resolution event-related potentials, Brain 127, 351–362. [DOI] [PubMed] [Google Scholar]