Abstract
Background and objectives
Preterm birth relates to long-term alterations in cardiac morphology and function. Understanding whether preterm postnatal life is a tractable period of cardiovascular development that can be positively altered by nutrition is relevant to long-term outcomes. We hypothesized that being fed human breast milk during early postnatal life is beneficial to long-term cardiac structure and function in preterm-born individuals compared with infant formulas.
Methods
A total of 926 preterm-born infants originally took part in a randomized controlled trial of postnatal milk-feeding regimens between 1982 and 1985 across 5 different UK centers. Preterm-born individuals were randomly assigned to either breast milk donated by unrelated lactating women or nutrient-enriched formulas. We followed 102 individuals from this cohort: 30 of whom had been randomized to being fed exclusively human milk and 16 to being fed exclusively formula. As a comparison group, we recruited an additional 102 individuals born term to uncomplicated pregnancies. Cardiac morphology and function were assessed by MRI.
Results
Preterm-born individuals fed exclusively human milk as infants had increased left and right ventricular end-diastolic volume index (+9.73%, P = .04 and +18.2%, P < .001) and stroke volume index (+9.79%, P = .05 and +22.1%, P = .01) compared with preterm-born individuals who were exclusively formula fed as infants.
Conclusions
This study provides the first evidence of a beneficial association between breast milk and cardiac morphology and function in adult life in those born preterm and supports promotion of human milk for the care of preterm infants to reduce long-term cardiovascular risk.
Increased survival of preterm infants has resulted in growing numbers now reaching adulthood. Recently we demonstrated these young adults exhibit a unique, adverse cardiac morphology and function, with reduced ventricular volumes and function and disproportionately increased mass.1,2 Experimental models implicate the early postnatal period as a key developmental window during which cardiac changes emerge. Preterm birth and the subsequent ex utero environment result in abnormal myocardial maturation, hypertrophy, and fibrosis.3,4 We therefore hypothesized different nutritional exposures during this period might modify preterm cardiac development. In particular, human milk feeding has been shown to preferentially influence the cardiovascular phenotype, which may in part relate to bioactive factors in breast milk.5–7 Therefore, we reanalyzed our data on cardiac morphology and function to take into account exposure to different milk feeding regimens.
Methods
Participants
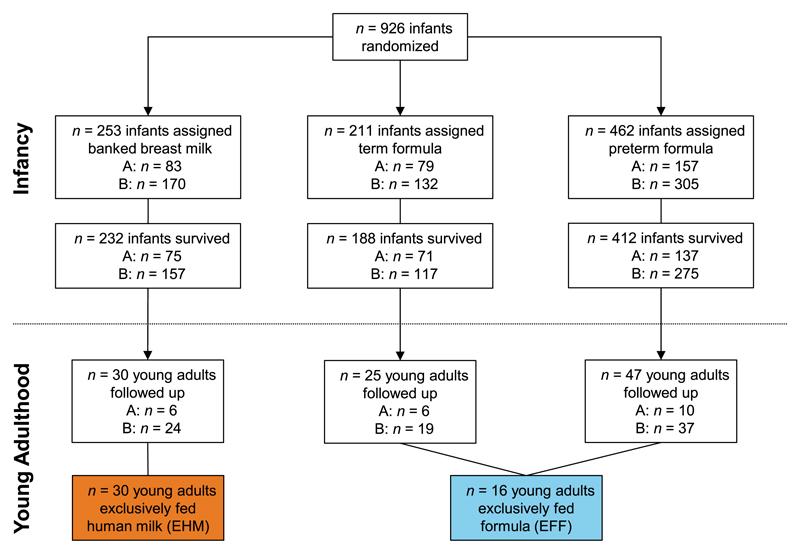
The study participants were recruited from a cohort of 926 preterm-born infants who participated in a randomized controlled trial, initially investigating the effects of early diet on later cognitive function (Fig 1). Details of the trial have been published comprehensively elsewhere,8 but briefly, infants born between 1982 and 1985 with no major congenital anomalies and of birth weight <1850 g were recruited in 5 UK centers. They were randomly assigned to either breast milk donated by unrelated lactating women, nutrient-enriched preterm formula (Farley’s Osterprem), or standard term formula (Farley’s Ostermilk; Farley's Health Products, Plymouth, UK). Within each trial, the diets were randomly assigned in 2 strata: the trial diets alone (A) and, in mothers who elected to express their own milk, the trial diets were assigned as supplements to mother’s milk (B; Fig 1). The composition of the assigned diets has previously been described.8,9 Follow-up studies were designed to test the hypothesis that early diet influences risk factors for cardiovascular disease.10
Figure 1.
Derivation of preterm-born study population followed up at age 23 to 28 years. A, infants receiving assigned milk as sole diet; B, infants receiving assigned milk as supplement to mother’s expressed milk.
A total of 102 of these subjects, aged between 23 and 28 years, were able to attend an appointment in Oxford for detailed cardiovascular phenotyping, including cardiac MRI (Fig 1).1,2 Of these, 30 individuals had been randomized to exclusively human milk (EHM) in the initial feeding trial, whereas the remaining 62 were randomized to either term formula or preterm formula. As we wished to study only those individuals who received the assigned milk as sole diet, 16 of the 62 were included as being exclusively fed formula (EFF). We recruited 102 young adults born term to uncomplicated pregnancies with age and sex distributions similar to those of the preterm-born young adults via advertisement to undergo identical investigations. All data were coded with subject- and study-specific IDs to ensure anonymity and blinded analysis. The follow-up study was registered with ClinicalTrials.gov (NCT01487824) and the protocol and recruitment strategy have previously been reported.1,2,11–13 The study was approved by the relevant ethics committee (Oxfordshire Research Ethics Committee A: 06/Q1604/118) and all participants provided signed informed consent.
Follow-up Visit in Young Adulthood
Anthropometry, Blood Pressure, Blood Samples, and Lifestyle Questionnaire
Subjects attended the Oxford Cardiovascular Clinical Research Facility in the morning after a 12-hour overnight fast. Anthropometric data, blood pressure measures, and blood sample collection were done as previously described.1,2,11–13 Data on medical history, smoking, parental medical history, and lifestyle were obtained by using a validated questionnaire.14
Cardiovascular Magnetic Resonance
Cardiovascular magnetic resonance was performed on a 1.5-T Siemens Sonata scanner (Siemens Healthcare, Erlangen, Germany).1,2 Steady-state free precession cine sequences were used to acquire localization images followed by optimized left ventricular horizontal and vertical long-axis cines. From these, a left ventricular short-axis cine stack was obtained with standardized basal slice alignment with a 7-mm slice thickness and 3-mm interslice gap. The cine images were stored on a digital archive for postprocessing, which was undertaken as detailed later in this article. We also acquired ShMOLLI T1 maps by using previously described methods15 and assessed thoracic dimensions and pulmonary artery diameters16 by using our localizer images (see Supplemental Information).
Quantification of Cardiac Geometry and Mass
Image analysis for right and left ventricular volumes, mass, and dimensions was performed offline by using Argus (Siemens Healthcare, Erlangen, Germany) as previously described.1,2 Briefly, left and right ventricular short-axis epicardial and endocardial borders were manually contoured for each slice at end-diastole and endocardial borders at end-systole to allow automated calculation of right and left ventricular mass and volumes, indexed to body surface area. Left ventricular internal cavity diameters were measured on the mid-ventricular short-axis slice at end-diastole while left ventricular lengths and right ventricular diameters were measured on the horizontal long-axis cines.
Assessment of Left Ventricular Geometry
We have previously created left ventricular statistical meshes for this population by using novel, in-house methods17,18 that have been made available to the scientific community at amdb.isd.kcl.ac.uk.19 Principal component analysis was undertaken to identify the key modes of variation of the shape between groups.
Statistical Analysis
Statistical analysis was carried out by using SPSS Version 22 (IBM SPSS Statistics, IBM Corporation, Chicago, IL). Normality of variables was assessed by visual assessment of normality curves and Shapiro-Wilk test. Comparison between groups for continuous variables was performed by using a 2-sided, independent-samples Student’s t test. For categorical variables, comparison was done by using a χ2 test. Pearson correlations (r) were used for bivariate associations and unstandardized regression coefficients (B) were used for bivariable and multivariable linear regression models. Results are presented as mean ± SD. P values were adjusted by using the Bonferroni method by multiplying the respective unadjusted P values by 3 (the number of pairwise comparisons when there are 3 groups) where multiple comparisons were performed between groups. Comparisons between groups were also adjusted for age and sex. P <.05 was considered statistically significant.
Results
Characteristics of Cohorts
There were no significant differences between preterm-born young adults who had been fed EHM as infants compared with those exclusively fed EFF in perinatal characteristics (Supplemental Table 3) or anthropometrics, demographics, blood biochemistry, or blood pressure in young adulthood (Table 1). However, both groups had altered blood biochemistry profiles and increased blood pressures compared with young adults born term. There were no significant differences in number of smokers, personal and family medical history, or lifestyle factors, such as socioeconomic status, physical activity, or diet (P > .05) between the 2 milk-feeding preterm groups, nor were there any differences between these groups compared with the control groups of adults born at term to uncomplicated pregnancies (Table 1 and Supplemental Table 4).
Table 1. Characteristics of Cohorts.
| Demographics and Anthropometrics | Preterm-Born EFF, n = 16 | Preterm-Born EHM, n = 30 | Pa | Term-Born Controls, n = 102 | Pb | Pc |
|---|---|---|---|---|---|---|
| Gestational age, wk | 29.7 ± 2.5 | 30.8 ± 2.3 | .22 | 39.6 ± 0.9 | <.001* | <.001* |
| Maternal preeclampsia, n (%) | 4 (25) | 8 (26.7) | >.99 | 0 (0) | >.99 | >.99 |
| Age, y | 24.8 ± 1.5 | 25.4 ± 1.4 | .42 | 25.0 ± 2.6 | >.99 | >.99 |
| Boys, n (%) | 7 (43.8) | 14 (46.7) | >.99 | 47 (46.1) | >.99 | >.99 |
| Smokers, n (%) | 4 (25.0) | 8 (26.7) | >.99 | 20 (19.6) | >.99 | >.99 |
| Birth weight, g | 1250.5 ± 309.2 | 1365.3 ± 257.8 | .41 | 3460.0 ± 417.0 | <.001* | <.001* |
| BMI | 24.4 ± 8.1 | 24.9 ± 4.1 | >.99 | 22.9 ± 3.1 | .26 | .08 |
| Height, m | 1.70 ± 0.09 | 1.70 ± 0.09 | >.99 | 1.74 ± 0.09 | .18 | .12 |
| Weight, kg | 69.7 ± 16.9 | 71.5 ± 12.5 | >.99 | 69.3 ± 12.5 | >.99 | >.99 |
| Body surface area, m2 | 1.81 ± 0.23 | 1.83 ± 0.18 | >.99 | 1.83 ± 0.20 | >.99 | >.99 |
| Waist:Hip | 0.76 ± 0.06 | 0.82 ± 0.07 | .38 | 0.81 ± 0.06 | .19 | .89 |
| Biochemistry | ||||||
| Total cholesterol, mmol/L | 5.23 ± 0.98 | 4.69 ± 0.89 | .44 | 4.23 ± 0.86 | .003* | .04* |
| HDL-C, mmol/L | 1.72 ± 1.5 | 1.50 ± 0.25 | .65 | 1.47 ± 0.41 | >.99 | >.99 |
| LDL-C, mmol/L | 2.87 ± 1.14 | 2.76 ± 0.77 | >.99 | 2.37 ± 0.66 | .05* | .04* |
| Triglycerides, mmol/L | 0.91 ± 0.35 | 0.93 ± 0.44 | >.99 | 0.87 ± 0.40 | >.99 | >.99 |
| Glucose, mmol/L | 4.81 ± 0.43 | 5.07 ± 0.41 | .32 | 4.61 ± 0.30 | .05* | .003* |
| Insulin, pmol/L | 59.8 ± 32.8 | 61.2 ± 36.9 | .57 | 35.6 ± 15.9 | <.001* | <.001* |
| Blood pressure, mm Hg | ||||||
| Systolic | 119.6 ± 11.3 | 121.0 ± 9.3 | >.99 | 112.9 ± 10.1 | .03* | <.001* |
| Diastolic | 72.3 ± 4.5 | 72.8 ± 8.2 | >.99 | 68.8 ± 7.0 | .14 | .02* |
| Mean arterial pressure | 88.0 ± 5.7 | 88.9 ± 7.2 | >.99 | 83.5 ± 7.1 | .03* | <.001* |
| Pulse pressure | 47.4 ± 8.2 | 48.2 ± 9.9 | >.99 | 44.1 ± 8.5 | .05* | .03* |
Values are mean ± SD unless stated otherwise. P values were adjusted by using the Bonferroni method for multiple group comparisons (3 groups). HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Preterm-born EHM versus preterm-born EFF. Comparisons adjusted for age and sex.
Preterm-born EFF versus term-born controls. Comparisons adjusted for sex.
Preterm-born EHM versus term-born controls. Comparisons adjusted for sex.
P < .05.
Left Ventricular Size and Function
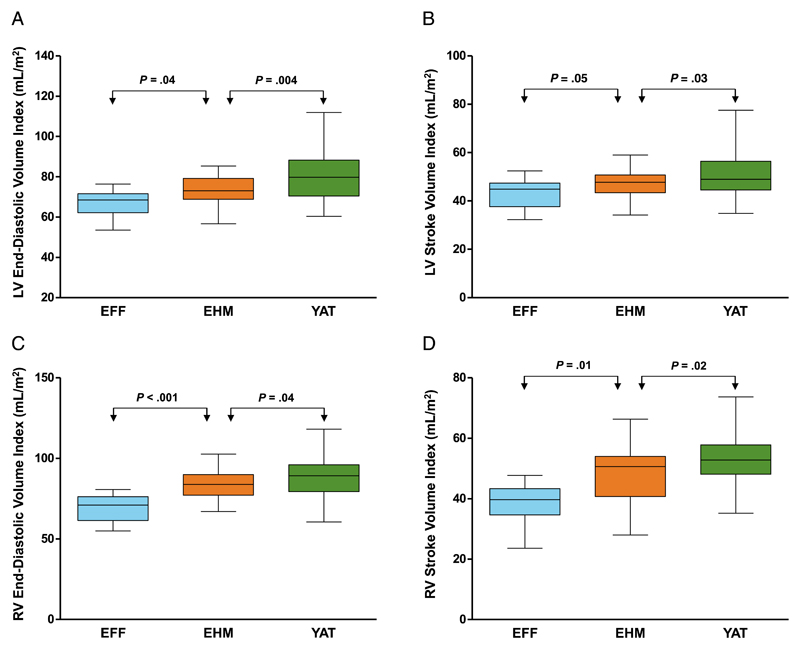
Preterm-born individuals fed EHM as infants have an increased mean left ventricular end-diastolic volume index (+9.73%; 73.3 ± 7.6 vs 66.8 ± 6.7 mL/m2, P = .04) and left ventricular stroke volume index (+9.79%; 47.1 ± 5.9 vs 42.9 ± 6.3 mL/m2, P = .05) compared with preterm-born individuals who were fed EFF as infants (Fig 2 and Table 2). Although both feeding groups still had significantly smaller left ventricular end-diastolic volume index and stroke volume index than adults born term, the reduction in the group fed EHM was only ~9% compared with 18% in the group fed EFF for both left ventricular end-diastolic volume index and stroke volume index. Both preterm-born groups had similar left ventricular mass index and ejection fraction (P > .99), although the preterm group fed EFF had significantly shorter left ventricles (P = .05) with reduced left ventricular luminal diameters (P = .009). The range of values for the left ventricular parameters in the term-born controls is similar to normal ranges previously published by using cardiovascular magnetic resonance.20
Figure 2.
Preterm-born young adults fed EHM as infants (orange) had increased left and right ventricular end-diastolic volume index (A and C), and left and right ventricular stroke volume index (B and D) compared with preterm-born young adults who were EFF as infants (blue). Term-born young adults (YAT) are shown in green. Please refer to Lewandowski et al.1 for an illustration and description of the modes of anatomical variation.
Table 2. Cardiac Parameters.
| Preterm-Born EFF, n = 16 | Preterm-Born EHM, n = 30 | Pa | Term-Born Controls, n = 102 | Pb | Pc | |
|---|---|---|---|---|---|---|
| Left ventricle | ||||||
| End-diastolic volume index, mL/m2 | 66.8 ± 6.7 | 73.3 ± 7.6 | .04* | 80.2 ± 11.7 | .003* | .004* |
| End-systolic volume index, mL/m2 | 23.9 ± 5.7 | 26.3 ± 5.6 | >.99 | 29.1 ± 6.4 | .03* | .03* |
| Stroke volume index, mL/m2 | 42.9 ± 6.3 | 47.1 ± 5.9 | .05* | 51.3 ± 8.9 | .02* | .03* |
| Ejection fraction, % | 64.3 ± 7.5 | 64.3 ± 5.7 | >.99 | 64.1 ± 4.9 | >.99 | >.99 |
| Mass index, g/m2 | 65.9 ± 11.1 | 66.4 ± 10.7 | >.99 | 55.4 ± 11.4 | <.001* | <.001* |
| Mass/end-diastolic volume | 0.95 ± 0.16 | 0.91 ± 0.15 | .70 | 0.70 ± 0.12 | <.001* | <.001* |
| Length, cm | 8.78 ± 0.47 | 9.28 ± 0.62 | .05* | 9.81 ± 0.73 | <.001* | .001* |
| Luminal diameter, cm | 4.83 ± 0.40 | 5.34 ± 0.45 | .009* | 5.64 ± 0.48 | <.001* | .001* |
| Right ventricle | ||||||
| End-diastolic volume index, mL/2 | 70.8 ± 8.5 | 83.7 ± 9.7 | <.001* | 88.5 ± 11.8 | <.001* | .04* |
| End-systolic volume index, mL/m2 | 32.0 ± 4.8 | 35.2 ± 7.1 | .15 | 35.6 ± 7.7 | .54 | >.99 |
| Stroke volume index, mL/m2 | 39.8 ± 7.6 | 48.6 ± 9.1 | .01* | 52.9 ± 7.2 | <.001* | .02* |
| Ejection fraction, % | 54.5 ± 7.1 | 57.8 ± 7.9 | .15 | 60.0 ± 5.3 | <.001* | .31 |
| Mass index, g/m2 | 24.2 ± 3.7 | 24.8 ± 3.0 | >.99 | 20.4 ± 3.4 | <.001* | <.001* |
| Mass/end-diastolic volume | 0.35 ± 0.06 | 0.29 ± 0.05 | .002* | 0.23 ± 0.03 | <.001* | <.001* |
| Length, cm | 8.06 ± 0.75 | 8.55 ± 0.69 | .10 | 8.97 ± 0.76 | <.001* | .001* |
| Luminal diameter, cm | 3.92 ± 0.42 | 4.34 ± 0.41 | .11 | 4.63 ± 0.55 | <.001* | .003* |
Values are mean ± SD unless stated otherwise. P values were adjusted by using the Bonferroni method for multiple group comparisons (3 groups).
Preterm-born EHM versus preterm-born EFF. Comparisons adjusted for age and sex.
Preterm-born EFF versus term-born controls. Comparisons adjusted for sex.
Preterm-born EHM versus term-born controls. Comparisons adjusted for sex.
P < .05.
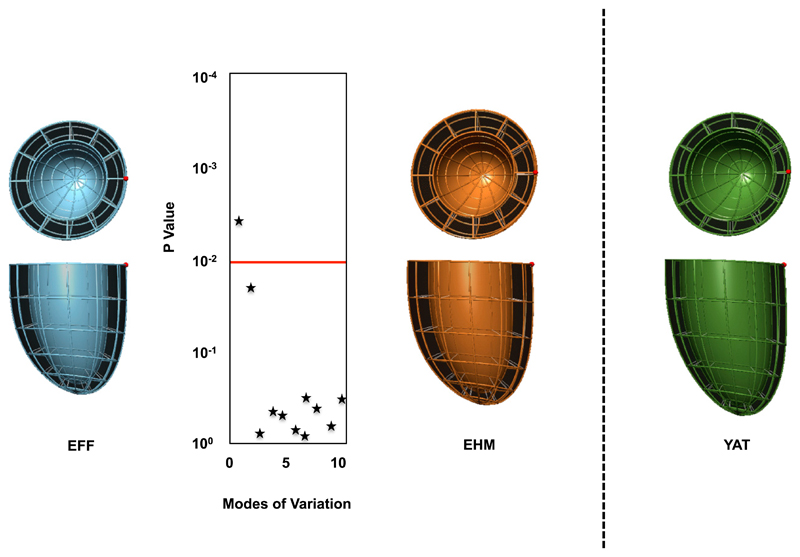
Left Ventricular Shape and Native T1 Imaging
Shape analysis confirmed the differences in size observed by using standard metrics (Fig 3), which particularly highlighted the significant increase in left ventricular length (P < .001) in the EHM group compared with the preterm individuals fed EFF. Importantly, there were no other major geometric variations identified between preterm-born individuals fed EHM and those fed EFF (Fig 3) or term-born adults. We did not observe significant differences in myocardial tissue characterization by T1 mapping. Preterm-born young adults fed EFF had similar native T1 values to those fed EHM (961.0 ± 43.1 vs 959.0 ± 27.3 ms, P > .99), and term-born young adults (969.1 ± 24.7 ms, P = .26 and P = .51, respectively), in range of the normal population values.15
Figure 3.
The statistical average shapes of the left ventricle are shown across each group derived from computational atlas formation. Statistical comparisons across the first 10 modes for the preterm-born young adults who were EFF (blue) and fed EHM (orange) are shown, with mode 1 being the key differentiating mode between groups (P < .001). Term-born young adults (YAT) are shown in green.
Right Ventricular Size and Function
Interestingly, the percentage differences between preterm-born young adults who had been fed EHM as infants compared with those fed EFF were much greater for right ventricular end-diastolic volume index (+18.2%, P < .001) and right ventricular stroke volume index (+22.1%, P = .01). Furthermore, although there was only a 5.42% reduction in right ventricular end-diastolic volume index and 8.13% in right ventricular stroke volume index in preterm-born young adults fed EHM compared with term-born controls, preterm-born young adults fed EFF showed substantially greater reductions in these cardiac parameters when compared with term-born controls (25.0% and 24.8%, respectively).
Right ventricular mass index and length were similar between preterm groups (Table 2). However, preterm-born young adults fed EHM had similar right ventricular ejection fractions as term-born young adults (P = .31), whereas preterm-born young adults fed EFF showed reduced right ventricular ejection fractions compared with term-born controls (P < .001). The range of values for the right ventricular parameters in the term-born controls is similar to normal ranges previously published by using cardiovascular magnetic resonance.20
Predictors of Cardiac Changes
We have previously demonstrated that gestational age is the strongest predictor of left and right ventricular end-diastolic volumes and stroke volumes.1,2 To test whether being fed EHM was also an independent predictor of these cardiac parameters in preterm-born individuals, we created separate multivariable regression models for each of our 4 major cardiac changes and included those fed EFF or EHM postnatally. In multivariable models including gestational age, sex, postnatal milk-feeding group, and postnatal weight gain, gestational age remained an independent predictor for all 4 cardiac measures (P < .001); however, being fed EHM was also an independent predictor of left ventricular end-diastolic volume index (P = .007), left ventricular stroke volume index (P = .03), right ventricular end-diastolic volume index (P = .002), and right ventricular stroke volume index (P = .004). In line with the changes between preterm feeding groups being greater for the right ventricle, in a multivariable regression model across the entire preterm group (n = 102) with gestational age, sex, percentage of human milk in the diet, and postnatal weight gain as the predictors, the percentage of human milk was positively related to both right ventricular end-diastolic volume index (P = .009) and stroke volume index (P = .04), but not left ventricular parameters. There were no correlations between percentage of expressed milk and cardiac measures within the group fed EHM.
Thoracic Cavity Size and Pulmonary Artery Diameters
Preterm-born young adults fed EFF had reduced thoracic cavity dimensions compared with those fed EHM (Supplemental Table 5). Although there were no differences between preterm-born young adults fed EFF versus EHM in aortic diameters (2.35 ± 0.30 vs 2.42 ± 0.22 cm, P = .36), those in the EFF group had increased main pulmonary artery diameters (2.41 ± 0.21 vs 2.16 ± 0.23 cm, P = .002) and pulmonary artery to aortic diameter ratios (1.04 ± 0.12 vs 0.89 ± 0.07, P < .001). Furthermore, pulmonary artery to aortic diameter ratios were inversely related to right ventricular end-diastolic volume index and stroke volume index (r = –0.41, P = .005 and r = –0.40, P = .007, respectively) but not left ventricular parameters.
Discussion
We provide the first evidence of an association between early postnatal nutrition in preterm-born infants and cardiac structure and function in later life. Left and right ventricular end-diastolic and stroke volumes in the EHM group approached values seen in term-born controls with particularly striking findings for the right ventricle.
Breast milk contains a greater bioactivity and bioavailability of a number of growth factors, enzymes, and antibodies compared with even the best infant formulas.21,22 These factors are relevant to normal growth and development, as well as improving preterm infant health. Although the content of preterm formulas in particular has been modified to better meet the needs of the growing preterm infant since the 1980s, such as the inclusion of polyunsaturated fatty acids,23 the American Academy of Pediatrics continues to recommend that preterm infants be given pasteurized donor milk rather than preterm formula if a mother is unable to provide adequate breast milk volume.24 The benefits of human breast milk have also been demonstrated to extend to other parts of the cardiovascular system. Human milk intake in preterm infants shows a beneficial association with proximal cerebral arterial vessel tortuosity: a marker of cerebrovascular development that is reduced in preterm infants.5 Breast milk may act through similar pathways to protect vascular and cardiac development as it contains essential growth factors, such as vascular endothelial growth factor, which are of particular benefit to early stages of vasculogenesis and angiogenesis in preterm infants.25 Altered vasculogenesis and angiogenesis in preterm individuals has been shown as early as fetal life and extends into adulthood,13,26 and is an important contributor to poor lung development in preterm infants, as it plays a pivotal role in alveolarization.27 It is therefore possible that, due to the interdependence of the lungs and the right ventricle, the reason we see greater benefits in right ventricular volumes and function in preterm-born young adults who were fed EHM postnatally as compared with the left ventricle is due to essential vascular growth factors contained in breast milk that benefit lung function and development.28 Although we did not collect lung function data in this cohort, the slight reduction in thoracic cavity dimensions and the increase in pulmonary artery size in the group fed EFF supports this notion, as does the close relationship between pulmonary artery dimensions and right ventricular morphology. The data suggest that breast milk may reduce the risk of pulmonary arterial hypertension and future cardiopulmonary disease in preterm-born individuals.16
As our study participants were still relatively young, continued follow-up will be necessary to assess clinical outcome as they reach later adulthood. Data on the relevance of cardiac morphology and function to disease development are sparse in young populations. Nevertheless, reductions in end-diastolic volume and stroke volume, which were much greater in the group fed EFF, lead to proportional changes in maximal exercise capacity29: an independent predictor of cardiovascular morbidity and mortality.30,31 Furthermore, the reductions in right ventricular systolic function in the group fed EFF are of independent and additive prognostic value in chronic heart failure and are powerful predictors of mortality in left heart failure,32 and may therefore directly contribute to the onset of clinical heart failure.33 We have not performed late gadolinium enhancement (LGE) imaging in this cohort to assess for scar burden. However, native T1 values were within the normal range,15 with no statistical differences between the groups studied. Given the lack of significantly elevated T1 values typically seen in areas of infarction34,35 and the lack of regional wall motion abnormalities, it is unlikely that large areas of focal scarring are missed; although small areas of scarring cannot be ruled out with absolute certainty, this is unlikely to alter the main message of the article.
Use of our computational atlas allowed us to confirm that cardiac differences between groups were proportional size changes, rather than alterations in geometric dimensions. These findings suggest the changes in cardiac morphology observed in preterm-born individuals relate primarily to size rather than geometric alterations, and that breast milk leads to proportional normalization of size. Interestingly, fetal growth–restricted offspring, who have more spherical hearts in fetal and postnatal life, also showed benefits of human breast milk, with longer breastfeeding durations relating to normalization of left ventricular sphericity.7 Although breast milk appears to have differing effects in these offspring, the findings support the necessity of breast milk as part of early nutrition for individuals born to pregnancy complications as a protective mechanism to cardiovascular development.
Our finding that gestational age remained a significant, independent predictor of cardiac parameters when milk-feeding diets were taken into account is in line with our previous results.1,2 Although our data suggest that it is not possible to reverse all cardiac changes related to the degree of prematurity through breast milk feeding, the magnitude of the differences between preterm-born adults fed EHM and those fed EFF is substantial and occurs independent of gestational age. Our finding that percentage of human milk consumed was positively associated with right ventricular morphology and function suggests that even small amounts of human milk in the preterm postnatal diet may be beneficial. Understanding the benefits and optimum duration of breast milk feeding on cardiac remodeling across a large range of gestational ages of preterm-born individuals will be of benefit to better understand feeding approaches for different gestational age categories of preterm birth.
Despite the magnitude of the cardiac differences between milk-feeding groups, this study does not prove causality. First, the number of participants available for comparison is relatively small compared with the size of the original cohort. Due to loss of follow-up and the long-term prospective nature of our study, the study was not fully randomized, and as such, there is risk of ascertainment bias. It is therefore possible that other associated factors in the perinatal period account for a proportion of the differences we have identified, such as genetic risk, epigenetic changes, and environmental factors.36,37 However, our preterm groups were similar in baseline demographics and demographic characteristics in young adulthood. Furthermore, unlike other studies that often rely on questionnaires and recall,7 we can be certain of the exact dietary intake of our preterm-born individuals during early postnatal life. It is possible that mothers who elect to breastfeed may be fundamentally different from mothers who do not. However, we found no evidence of this in our group fed EHM, suggesting that donor milk provides a similar level of benefit as the expressed breast milk from their mothers. The randomized preterm population was previously followed up at age 13 to 16 years,6 and in that study breast milk consumption had a beneficial impact on blood pressure in adolescents born preterm resulting in a 4-mm Hg lower mean arterial pressure. Our group was smaller and, as a result, our analysis was not powered to identify this size of difference in blood pressure, although was still able to identify the proportionally larger variation in cardiac morphology associated with breast milk consumption. Interestingly, the similar blood pressures and metabolic profiles between groups does support the concept that the differences between EHM and EFF preterm-born young adults occur independently of other cardiovascular risk factors. Longer-term follow-up will be required to understand the true impact of this variation on cardiovascular risk.
Conclusions
We provide the first evidence of a beneficial association between breast milk and cardiac morphology in adult life in those born preterm. The findings implicate early preterm postnatal life as a potentially tractable period of cardiovascular development, relevant to long-term outcomes, and support promotion of human milk for the care of preterm infants to reduce long-term cardiovascular risk.
Supplementary Material
What’s Known on this Subject
Preterm-born young adults exhibit an adverse cardiac morphology and function. The preterm postnatal period is a key developmental window during which cardiac changes emerge, although it is not known whether postnatal nutrition is relevant to long-term cardiac structure and function.
What this Study Adds
This study provides the first evidence of a beneficial association between postnatal breast milk consumption and cardiac morphology in adulthood in preterm-born individuals. These findings support promotion of human milk for preterm infant care to reduce long-term cardiovascular risk.
Funding
Dr Lewandowski was funded by the Commonwealth Scholarship and Fellowship Program and a British Heart Foundation Project grant (PG/13/58/30397). This work was funded by grants to Dr Leeson from the British Heart Foundation (FS/06/024 and FS/11/65/28865). Dr Lamata holds a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant 099973/Z/12/Z). Dr Singhal was supported by Great Ormond Street Hospital Children's Charity. Additional grants were received from the National Institute for Health Research Oxford Biomedical Research Centre and Oxford British Heart Foundation Centre for Research Excellence. Previous cohort follow-up was supported by the Medical Research Council. Funders were not involved in the design or conduct of the study; the collection, management, or interpretation of the data; nor were they involved in the preparation, review, or approval of the manuscript.
Abbreviations
- EFF
exclusively fed formula
- EHM
exclusively human milk
Footnotes
Dr Lewandowski conceptualized and designed the follow-up study, carried out data collection and analysis, drafted the initial manuscript, and approved the final manuscript as submitted. Dr Lucas conceptualized and designed the initial milk-feeding trial, reviewed and revised the manuscript, and approved the final manuscript as submitted. Drs Neubauer and Leeson conceptualized and designed the follow-up study, reviewed and revised the manuscript, and approved the final manuscript as submitted. Drs Lamata, Piechnik, Ferreira and Boardman and Mrs Francis carried out data collection and analysis; Professor Singhal reviewed and revised the manuscript, and approved the final manuscript as submitted; the data were interpreted with input from all coauthors.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01487824).
Financial Disclosure: Dr Lucas is a consultant to Prolacta Bioscience; the other authors have indicated they have no financial relationships relevant to this article to disclose.
Potential Conflict of Interest: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Lewandowski AJ, Augustine D, Lamata P, et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation. 2013;127(2):197–206. doi: 10.1161/CIRCULATIONAHA.112.126920. [DOI] [PubMed] [Google Scholar]
- 2.Lewandowski AJ, Bradlow WM, Augustine D, et al. Right ventricular systolic dysfunction in young adults born preterm. Circulation. 2013;128(7):713–720. doi: 10.1161/CIRCULATIONAHA.113.002583. [DOI] [PubMed] [Google Scholar]
- 3.Bertagnolli M, Huyard F, Cloutier A, et al. Transient neonatal high oxygen exposure leads to early adult cardiac dysfunction, remodeling, and activation of the renin-angiotensin system. Hypertension. 2014;63(1):143–150. doi: 10.1161/HYPERTENSIONAHA.113.01760. [DOI] [PubMed] [Google Scholar]
- 4.Bertagnolli M, Luu TM, Lewandowski AJ, Leeson P, Nuyt AM. Preterm birth and hypertension: is there a link? Curr Hypertens Rep. 2016;18(4):28. doi: 10.1007/s11906-016-0637-6. [DOI] [PubMed] [Google Scholar]
- 5.Vasu V, Durighel G, Thomas EL, et al. Preterm nutritional intake and MRI phenotype at term age: a prospective observational study. BMJ Open. 2014;4(5):e005390. doi: 10.1136/bmjopen-2014-005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singhal A, Cole TJ, Lucas A. Early nutrition in preterm infants and later blood pressure: two cohorts after randomised trials. Lancet. 2001;357(9254):413–419. doi: 10.1016/S0140-6736(00)04004-6. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Lopez M, Osorio L, Acosta-Rojas R, et al. Influence of breastfeeding and postnatal nutrition on cardiovascular remodeling induced by fetal growth restriction. Pediatr Res. 2016;79(1–1):100–106. doi: 10.1038/pr.2015.182. [DOI] [PubMed] [Google Scholar]
- 8.Lucas A, Gore SM, Cole TJ, et al. Multicentre trial on feeding low birthweight infants: effects of diet on early growth. Arch Dis Child. 1984;59(8):722–730. doi: 10.1136/adc.59.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas A, Morley R, Cole TJ. Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ. 1998;317(7171):1481–1487. doi: 10.1136/bmj.317.7171.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas A, Morley R. Does early nutrition in infants born before term programme later blood pressure? BMJ. 1994;309(6950):304–308. doi: 10.1136/bmj.309.6950.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly BA, Lewandowski AJ, Worton SA, et al. Antenatal glucocorticoid exposure and long-term alterations in aortic function and glucose metabolism. Pediatrics. 2012;129(5) doi: 10.1542/peds.2011-3175. Available at: www.pediatrics.org/cgi/content/full/129/5/e1282. [DOI] [PubMed] [Google Scholar]
- 12.Lewandowski AJ, Lazdam M, Davis E, et al. Short-term exposure to exogenous lipids in premature infants and longterm changes in aortic and cardiac function. Arterioscler Thromb Vasc Biol. 2011;31(9):2125–2135. doi: 10.1161/ATVBAHA.111.227298. [DOI] [PubMed] [Google Scholar]
- 13.Lewandowski AJ, Davis EF, Yu G, et al. Elevated blood pressure in pretermborn offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension. 2015;65(3):607–614. doi: 10.1161/HYPERTENSIONAHA.114.04662. [DOI] [PubMed] [Google Scholar]
- 14.Leeson CPM, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation. 2001;103(9):1264–1268. doi: 10.1161/01.cir.103.9.1264. [DOI] [PubMed] [Google Scholar]
- 15.Piechnik SK, Ferreira VM, Lewandowski AJ, et al. Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J Cardiovasc Magn Reson. 2013;15(1):13. doi: 10.1186/1532-429X-15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truong QA, Massaro JM, Rogers IS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging. 2012;5(1):147–154. doi: 10.1161/CIRCIMAGING.111.968610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamata P, Sinclair M, Kerfoot E, et al. An automatic service for the personalization of ventricular cardiac meshes. J R Soc Interface. 2013;11(91):20131023. doi: 10.1098/rsif.2013.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamata P, Niederer S, Nordsletten D, et al. An accurate, fast and robust method to generate patient-specific cubic Hermite meshes. Med Image Anal. 2011;15(6):801–813. doi: 10.1016/j.media.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Kerfoot E, Lamata P, Niederer S, Hose R, Spaan J, Smith N. Share and enjoy: anatomical models database—generating and sharing cardiovascular model data using Web services. Med Biol Eng Comput. 2013;51(11):1181–1190. doi: 10.1007/s11517-012-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7(5):775–782. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 21.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60(1):49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schanler RJ, Hurst NM, Lau C. The use of human milk and breastfeeding in premature infants. Clin Perinatol. 1999;26(2):379–398. vii. [PubMed] [Google Scholar]
- 23.Fleith M, Clandinin MT. Dietary PUFA for preterm and term infants: review of clinical studies. Crit Rev Food Sci Nutr. 2005;45(3):205–229. doi: 10.1080/10408690590956378. [DOI] [PubMed] [Google Scholar]
- 24.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3) doi: 10.1542/peds.2011-3552. Available at: www.pediatrics.org/cgi/content/full/129/3/e827. [DOI] [PubMed] [Google Scholar]
- 25.Siafakas CG, Anatolitou F, Fusunyan RD, Walker WA, Sanderson IR. Vascular endothelial growth factor (VEGF) is present in human breast milk and its receptor is present on intestinal epithelial cells. Pediatr Res. 1999;45(5 pt 1):652–657. doi: 10.1203/00006450-199905010-00007. [DOI] [PubMed] [Google Scholar]
- 26.Ligi I, Simoncini S, Tellier E, et al. A switch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood. 2011;118(6):1699–1709. doi: 10.1182/blood-2010-12-325142. [DOI] [PubMed] [Google Scholar]
- 27.Thébaud B, Ladha F, Michelakis ED, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112(16):2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 28.Ogbuanu IU, Karmaus W, Arshad SH, Kurukulaaratchy RJ, Ewart S. Effect of breastfeeding duration on lung function at age 10 years: a prospective birth cohort study. Thorax. 2009;64(1):62–66. doi: 10.1136/thx.2008.101543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. JAMA. 2009;301(3):286–294. doi: 10.1001/jama.2008.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 31.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 32.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117(13):1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 33.Kawut SM, Barr RG, Lima JAC, et al. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi-Ethnic Study of Atherosclerosis (MESA)—right ventricle study. Circulation. 2012;126(14):1681–1688. doi: 10.1161/CIRCULATIONAHA.112.095216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu A, Wijesurendra RS, Francis JM, et al. Adenosine stress and rest T1 mapping can differentiate between ischemic, infarcted, remote, and normal myocardium without the need for gadolinium contrast agents. JACC Cardiovasc Imaging. 2016;9(1):27–36. doi: 10.1016/j.jcmg.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kali A, Choi E-Y, Sharif B, et al. Native T1 mapping by 3-T CMR imaging for characterization of chronic myocardial infarctions. JACC Cardiovasc Imaging. 2015;8(9):1019–1030. doi: 10.1016/j.jcmg.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Lewandowski AJ, Leeson P. Preeclampsia, prematurity and cardiovascular health in adult life. Early Hum Dev. 2014;90(11):725–729. doi: 10.1016/j.earlhumdev.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Davis EF, Lewandowski AJ, Aye C, et al. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. 2015;5(6):e008136. doi: 10.1136/bmjopen-2015-008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.