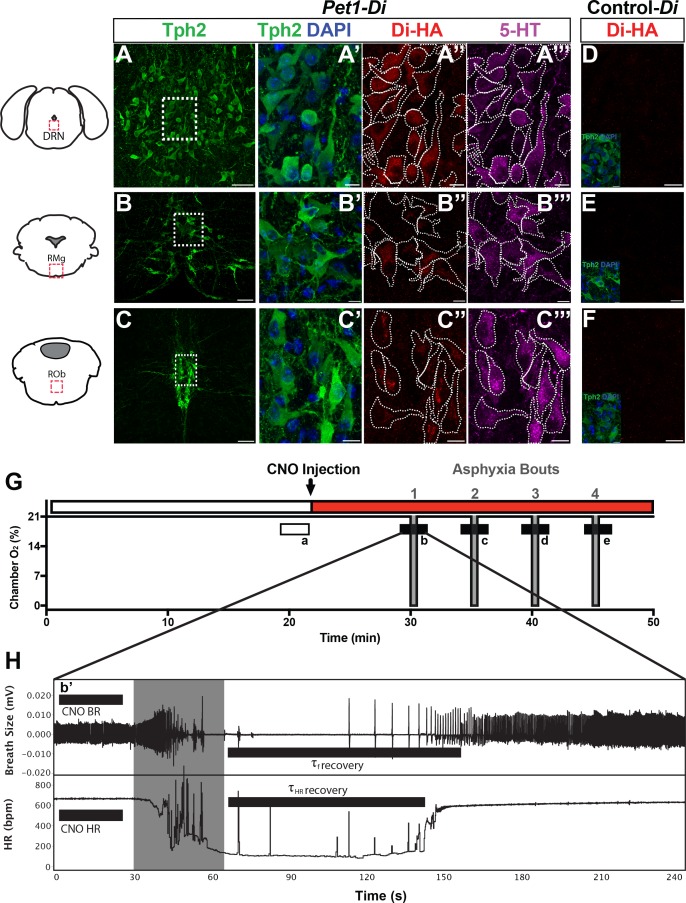
Figure 1. Genetic mouse model and postneonatal autoresuscitation assay.
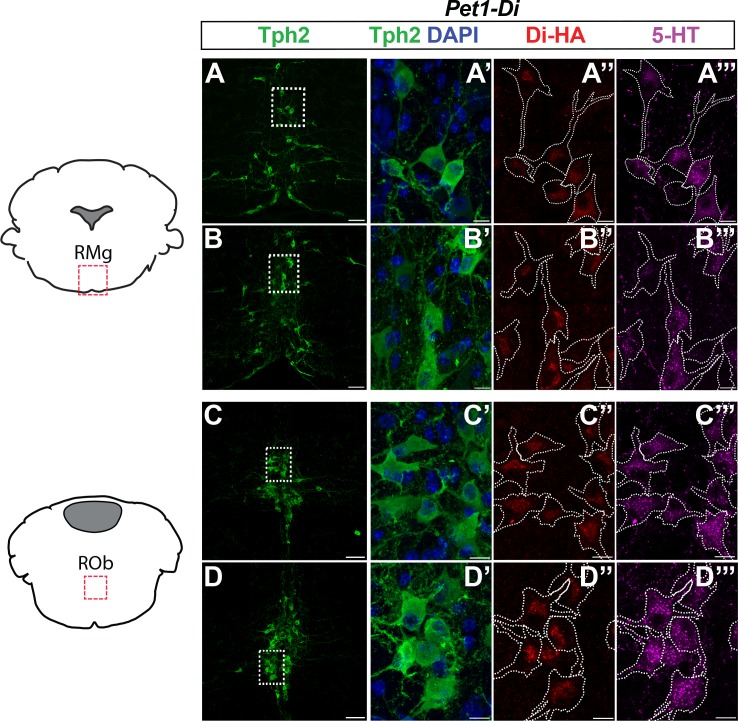
(A–C”’) HA-tagged Di receptor expression targeted to Pet1-raphe serotonergic neurons in double-transgenic Pet1-Flpe; RC-FDi (referred to as Pet1-Di) pups at P8, as shown previously in adult mice (Brust et al., 2014). (A–C) Low magnification view of 20 µm coronal section showing neurons immunopositive for tryptophan hydroxylase 2 (Tph2), identifying serotonergic neurons in the dorsal raphe nucleus (DRN) (A), the raphe magnus nucleus (RMg) (B), and the raphe obscurus (ROb) (C). Fields demarcated by dashed rectangles in A–C are shown at higher magnification in A’–C’”, with Tph2 immunoreactivity again in green (A’–C’), HA-Di immunopositivity in red (A”–C”), serotonin (5-HT) immunopositivity in magenta (A”’–C”’), and dashed cell outlines as grossly determined by the Tph2 immunodetection signal. The raphe location, proportion, and intensity of HA-Di immunodetection signal was qualitatively similar across Pet1-Di pups from independent litters (Figure 1—figure supplement 1). (D–F) Representative fields from negative-control single transgenics harboring the unrecombined RC-FDi allele (referred to as Control-Di), showing no detectable HA-Di, in line with prior validation that Di-expression from RC-FDi requires Flpe-recombination. Insets show Tph2 immunodetection of serotonergic neurons in these fields. Scale bars in A–C equal 50 µm, and in A’–C”” and D-F, 10 µm. (G–H) Schematic of repeated asphyxia-induced apnea and autoresuscitation recovery, during which breath size and heart rate are continuously monitored. (G) Plethysmograph chamber oxygen (O2) percent across assay time, starting with ~20 min of pup acclimation to chamber air (21% O2) including extraction of baseline cardiorespiratory values during the temporal window indicated by the open rectangle a. I.P. injection of CNO immediately follows; red rectangle indicates CNO exposure window. Asphyxia-apnea bouts are indicated by the four periods (b–e) of ~0% O2 (97% N2, 3% CO2) shown in gray. (H) Temporally expanded view of an asphyxia-apnea bout including example tracing of breath size (and calculated breathing rate, BR) and heart rate (HR) over time. Primes (b’ and similarly for c-e) indicate the bout-specific baseline post CNO injection immediately preceding asphyxia. The gray window indicates the period of asphyxia that induced the apnea, which is followed by immediate return to 21% O2 during which autoresuscitation recovery ensues. Black bars after asphyxia indicate τf or τHR (τ defined as 63% of the baseline value before that specific asphyxic bout). Light gray box indicates period of asphyxia (97% N 3% CO2) used to induce the apnea.