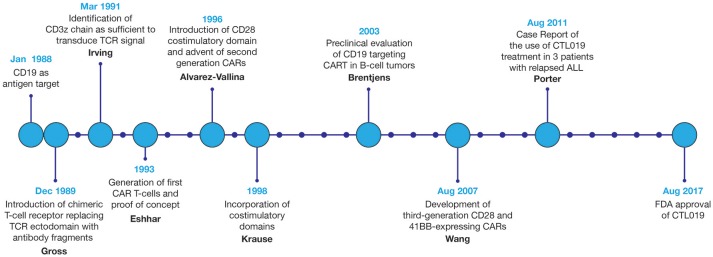
Figure 3.
Development of CART immunotherapy. Following development of the first chimeric T-cell receptor in 1989, early preclinical studies of the first CARTs demonstrated the ability to selectively identify and destroy antigen-expressing tumor cells (5, 6). However, upon adoptive transfer into live patients, T-cells expressing these first-generation CARs displayed limited persistence and were often rendered anergic due to the absence of costimulatory signals within the tumor microenvironment (TME) (26). With the introduction of costimulatory domains to provide these necessary activating signals, CART immunotherapy experienced a dramatic improvement in therapeutic efficacy (22). Optimization of CAR structure and ex vivo culture conditions to improve CART persistence, cytotoxicity, and resistance to tumor-induced immunosuppression remains an area of continued research. Evaluated in a variety of tumor types, CART immunotherapy has been markedly successful in the eradication of liquid tumors, culminating in the FDA approval of CART immunotherapy for the treatment of relapsed or refractory B-cell ALL in 2017.