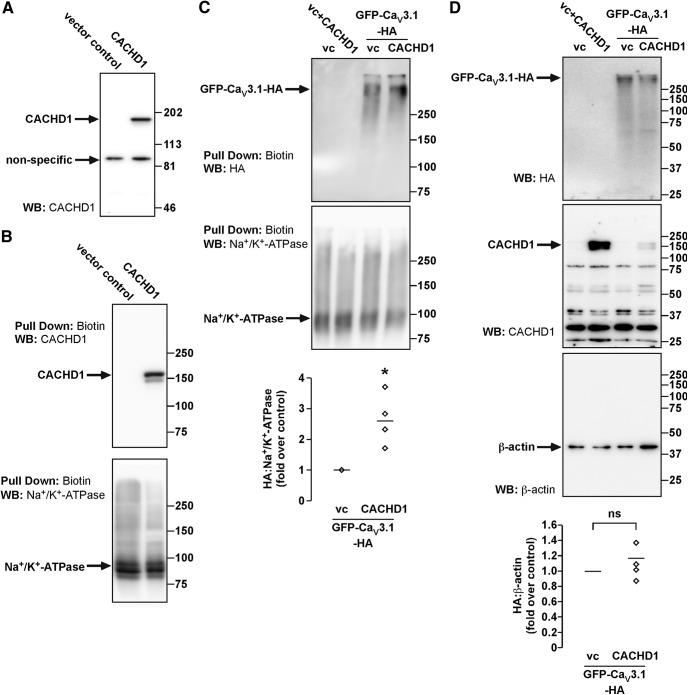
Figure 4.
Characterization of CACHD1 and its effects on CaV3.1 expression. HEK cells were transfected with empty vector (VC), CACHD1, Myc-CACHD1, and GFP-CaV3.1-HA alone or in combination, as shown in each panel. A, HEK cell lysates were analyzed by Western blotting (WB). An antibody to CACHD1 recognized a single protein similar to the predicted size for CACHD1, but also recognized a nonspecific protein in all lysates. B, Cell-surface proteins were biotinylated, and pull-downs were analyzed for CACHD1 and Na+/K+-ATPase (loading control). In control cells, no immunoreactive CACHD1 was detected, confirming antibody specificity. In CACHD1-expressing cells, immunoreactive CACHD1 was detected. In both cell types, immunoreactive Na+/K+-ATPase was detected. C, Cell-surface proteins were biotinylated, and pull-downs were analyzed for GFP-CaV3.1-HA (HA) and Na+/K+-ATPase (loading control). In control cells and cells only expressing CACHD1, no HA signals were detected, confirming antibody specificity. In cells expressing GFP-CaV3.1-HA, HA signals were readily detected. Quantification of the HA signals (normalized to Na+/K+-ATPase) revealed the expression of CACHD1-increased signals for GFP-CaV3.1-HA at the cell surface, *p < 0.05. Na+/K+-ATPase signals were detected in all cell types. D, Inputs of the biotin pull-down assays were analyzed by WB. Signals for HA were detected only in cells expressing GFP-CaV3.1-HA, signals for CACHD1 were detected only in cells expressing Myc-CACHD1, and signals for β-actin were detected in all cell types. All blots are representative of n ≥ 3 experiments.