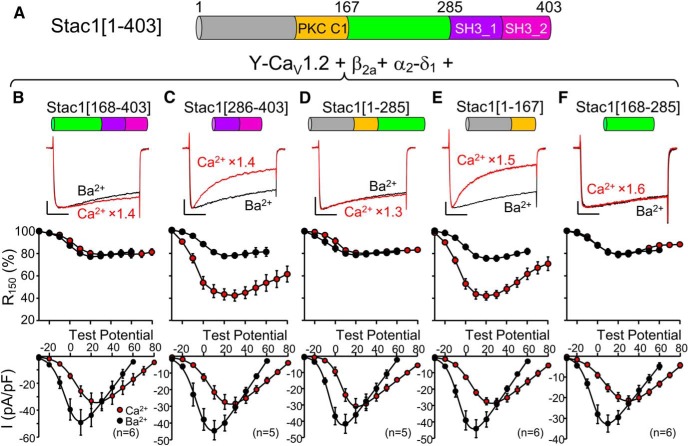
Figure 8.
The unstructured region of Stac1 (residues 168–285), which links the PKC C1 and SH3_1 domains, is sufficient to suppress calcium-dependent inactivation of CaV1.2. A, Schematic representation and domain architecture of full-length mouse Stac1 (residue numbers adapted from UniProt). B–F, for tsA201 cells transfected with CaV1.2, β2a, α2-δ1, and the indicated Stac1 construct. The top row illustrates representative peak currents carried by Ca2+ (red, vertically scaled by indicated factors, test potential of +30 mV except +20 mV for D) or Ba2+ (black, test potential of +10 mV), the middle row shows the fraction of peak current remaining 150 ms after the peak (R150) as a function of test potential, and the bottom row illustrates the peak I–V relationships. Scale bars: 10 pA/pF (unscaled Ba2+ currents, vertical), 50 ms (horizontal). Figure 8-1 and Figure 8-2 show that the region linking the PKC C1 and SH3_1 domains of both Stac2 and Stac3, respectively, is also sufficient to completely suppress calcium-dependent inactivation. Figure 8-3 shows that smaller, N- or C-terminal segments of this region either do not, or only partially, suppress calcium-dependent inactivation.