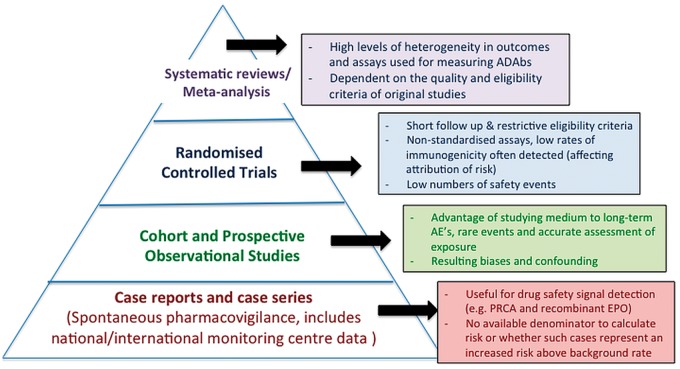
Fig. 2.
Hierarchy of evidence and assessment of drug safety in association with immunogenicity
Spontaneous pharmacovigilance data includes adverse event monitoring by the FDA and MHRA. While randomized controlled trials are the gold standard in the assessment of efficacy, prospective observational cohort studies provide the most useful information in the assessment of risk of reported safety events in association with immunogenicity. Each study design has its benefits and outlined limitations in the assessment of drug safety. AEs, adverse events; EPO, erythropoietin; PRCA, pure red cell aplasia.