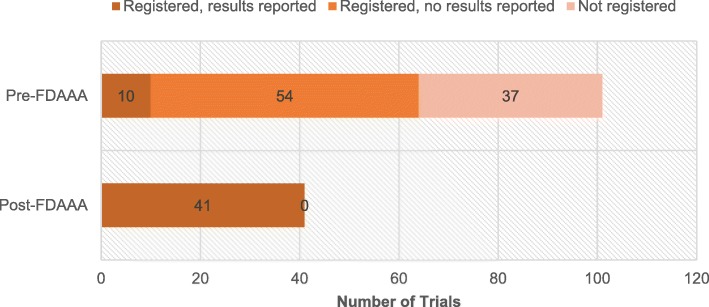
Fig. 2.
Registration and results reporting status of trials supporting FDA indications by FDAAA applicability, 2005–2014. Post-FDAAA trials were significantly more likely to be registered (100% vs 64%; p < 0.001) and report results (100% vs 10%; p < 0.001) than pre-FDAAA. Outcomes were compared by two-tailed Fisher exact tests