Abstract
Background and Objectives:
Procurement of tissue core biopsy samples may overcome some of the limitations of endoscopic ultrasound (EUS)-guided fine-needle aspiration. We aimed at assessing the safety, histological sample procurement yield, and diagnostic accuracy of a newly available histology needle.
Materials and Methods:
Data from consecutive patients with pancreatic solid lesions who underwent EUS-fine needle biopsy (EUS-FNB) using the 22-gauge Acquire™ needle were retrospectively retrieved from four tertiary care centers database.
Results:
Fifty-nine patients (mean age 68 ± 12 years; male/female 29/30) with pancreatic solid lesions underwent EUS-FNB using the 22-gauge Acquire™ needle. The biopsy was done transgastrically in 22 (37.3%) patients and transduodenally in 37 (62.7%) cases. A mean of 2.8 ± 0.45 needle passes per lesion site were performed, without any major complication. A tissue core biopsy sample for histological evaluation was obtained in 55 (93.2%) cases. In the additional four cases, the specimen obtained resulted adequate for cytological evaluation. Considering malignant versus nonmalignant disease, sensitivity, specificity, negative likelihood ratio, positive likelihood ratio, and diagnostic accuracy were 98.2% (95% confidence interval [CI], 90.6–99.7), 100% (95% CI, 43.6–100), 0.018 (95% CI, 0.003–0.125), 295.6 (95% CI, 0–9.3 × 1010), and 98.3% (95% CI, 94.9–100), respectively.
Conclusions:
EUS-FNB using the 22-gauge Acquire™ needle is able to reach a very high procurement yield and diagnostic accuracy. Large prospective studies are warranted to further evaluate the utility of this newly developed needle.
Keywords: Core biopsy, endoscopic ultrasound, fine-needle aspiration, fine-needle biopsy, histology, tissue acquisition
INTRODUCTION
In the last decade, in an attempt to overcome some of the limitations of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA), different techniques and specifically designed needles to acquire samples for histological evaluation have been developed.[1] One of the main limitations of EUS-FNA is the need for rapid on-site evaluation (ROSE) of the collected specimens to reach a diagnostic accuracy >90%.[2,3,4,5] However, the availability of ROSE is limited and has resulted in a perception of EUS as of limited utility,[6] while the lack of cytology expertise outside high volume tertiary care centers has resulted in a limited dissemination of the procedure in the community and in many countries.[7]
In centers where ROSE is not available, it has been recently recommended to perform EUS-fine needle biopsy (EUS-FNB) to acquire samples for histological evaluation.[8] Histological samples are easier to be interpreted by the pathologists, with the additional advantage of providing tissue cores to perform ancillary tests. The latter possibility is particularly important in view of the increasing interest in evaluating available samples for molecular markers, which may serve as prognostic predictors and targets for individualized chemotherapy in patients with cancer.[9,10] When this will occur, a transformation of diagnostic EUS into a more therapeutic procedure will take place and EUS will be performed not only to provide a diagnosis but also to offer the possibility to establish the best therapy for each individual patient.[11,12]
Among the available needles specifically designed to perform FNB, the 25-gauge Procore™ (Cook Medical, Bloomington, IN, USA) has been reported to gather tissue core samples in only about 40% of the cases.[13,14] Moreover, no clear advantages of the 22-gauge Procore™ (Cook Medical) over standard 22-gauge FNA needles have been demonstrated.[15] Finally, very promising results have been firstly reported for the 19-gauge Procore™, which were not followed by additional experiences.[16,17] On the other hand, a high accuracy of standard 19-gauge in acquiring tissue core biopsy samples for various indications has been reported.[18,19,20,21,22,23,24,25,26] However, it is not simple to utilize the 19-gauge needle, especially for transduodenal biopsy and the fear of complications reduced its use by nonexpert endosonographers. Based on these premises, a new needle for EUS-FNB, the Acquire™ needle (Boston Scientific Corporation, Marlborough, MA, USA), has become available in three different sizes, 25-gauge, 22-gauge, and 19-gauge. Preliminary data on the 22-gauge for both pancreatic and nonpancreatic lesions are encouraging.[27] However, no clear data of the clinical significance of these needles in a meaningful number of patients are available.
To answer this important question, we performed a retrospective evaluation of all sampling procedures performed using the 22-gauge Acquire™ needle in patients with pancreatic solid lesions.
MATERIALS AND METHODS
Patients
All consecutive patients with solid pancreatic lesions who, between May 2016 and August 2016, underwent EUS-FNB using the 22-gauge Acquire™ needle in four Italian centers were retrospectively retrieved from each single institution database.
The protocol to perform retrospective revision of the cases performed was approved by the Medical Ethics Committees. All patients gave their informed consent before the EUS-FNB procedure.
Study device
The Acquire™ needle has an outer diameter of 0.72 mm and an adjustable working length of 137.5–141.5 cm. The needle design has a crown tip with three symmetrical surfaces that manifest as cutting edges [Figure 1]. The needle geometry incorporates a smaller included angle and a larger inclination angle.
Figure 1.

The design of the tip of the newly developed Acquire™ needle with three symmetrical cutting surfaces with fully formed heels for precise cutting capabilities. Reproduced with the written permission from Boston Scientific Corporation
Endoscopic ultrasound sampling procedures
All EUS procedures were performed by advanced echoendoscopists, with the patients under conscious or deep sedation using a conventional linear EUS scope (GF-UC180T, Olympus Medical Systems Europe, or EG3870UTK, Pentax Europe GmbH, Hamburg, Germany). Once the pancreatic solid lesion was identified by EUS, an eligible puncture site without intervening vessels was selected. Puncture of the lesion using the 22-gauge Acquire™ needle was performed in all centers using the wet suction technique.[28] Briefly, in the wet suction technique, before puncturing the lesion, the stylet is removed and the needle is preflushed with 5 mL of saline to replace the column of air with fluid. A 10 cc syringe prefilled with 3 mL of normal saline is left attached to the proximal port and later used for aspiration after puncturing the lesion. Once the needle is passed into the lesion, the needle is moved to and fro three times followed by maximal strength suction to obtain the aspirate.
Multiple needle passes were performed in all patients and all the collected materials were placed directly in formalin for subsequent analyses. The formalin-fixed specimens were processed into paraffin according to standard routine methods. Sections of 5 μm were cut and stained with hematoxylin and eosin for conventional histology and with the proper immunostaining when necessary to reach a definitive diagnosis.
Histopatologic definitions
The procured sample was defined as a histological sample when an architecturally intact piece of tissue sufficient for histological evaluation of the targeted lesion was present and could be evaluated. A fragment that did not meet the criteria for architecturally intact histology but could still yield a diagnosis based on cell morphology was classified as a cytological sample.
Outcome measurements
Procurement yield was defined as the percentage of cases in which a histologically interpretable specimen could be retrieved. Diagnostic accuracy was defined by the rate of correct diagnosis obtained through analysis of the tissue samples acquired with Acquire™ needles. When examination of the acquired specimen was diagnostic for malignancy, this was considered the definitive diagnosis. For patients with a sample nondiagnostic for malignancy or for a specific benign disease, the presence or exclusion of malignancy was based on following criteria: the histopathological examination of the surgically resected specimen when available, the results of other diagnostic investigations such as computed tomography-guided and/or laparoscopic biopsy indicating the presence of malignancy and/or the long-term clinical follow-up, including follow-up imaging. For this purpose, these patients were evaluated for a minimum of 6 months.
Statistical analysis
Frequencies, percentages, means ± standard deviation, or medians with interquartile range were used, as appropriate, for descriptive analysis. Sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio were calculated. For the purpose of these analyses, definitive diagnoses were divided into malignant and nonmalignant cases. Technical failures or samples that were inadequate for histological evaluation were considered as false negative cases. Statistical analysis was performed using SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA) and WINPEPI version 11.39 (JH Abramson, October 2013, http://www.brixtonhealth.com/).
RESULTS
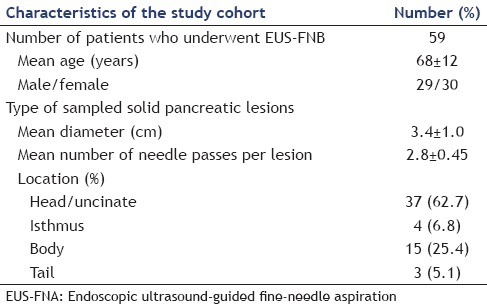
During the study period, 59 patients (mean age 68 ± 12 years; male/female 29/30) with pancreatic solid lesions underwent EUS-FNB using the 22-gauge Acquire™ needle. Their characteristics and the location of lesions, for which EUS-FNB was performed, are shown in Table 1. Among all the 59 pancreatic lesions evaluated, 37 (62.7%) were located in the uncinate process/head, 4 (6.8%) in the isthmus, 15 (25.4%) in the body, and 3 (5.1%) in the tail. The mean diameter of the lesions was 3.4 ± 1 cm. The biopsy was done transgastrically in 22 (37.3%) patients and transduodenally in 37 (62.7%) cases. A mean of 2.8 ± 0.45 needle passes per lesion site was performed, without any major complication [Table 1].
Table 1.
Characteristics of patients and indications for the performance of endoscopic ultrasound-guided fine-needle biopsy using the 22-gauge Aquire™ needles
A tissue core biopsy sample for histological evaluation was obtained in 55 (93.2%) cases. Representative cases are shown in Figure 2. In 3 (5.1%) additional cases, the material was scarce; while in the remaining patient (1.7%), no sample for histological examination was found. In these four cases, the specimen obtained resulted adequate for cytological evaluation. Finally, immunostainings to help reaching a definitive diagnosis were performed on tissue specimens in 44 cases (74.6%).
Figure 2.
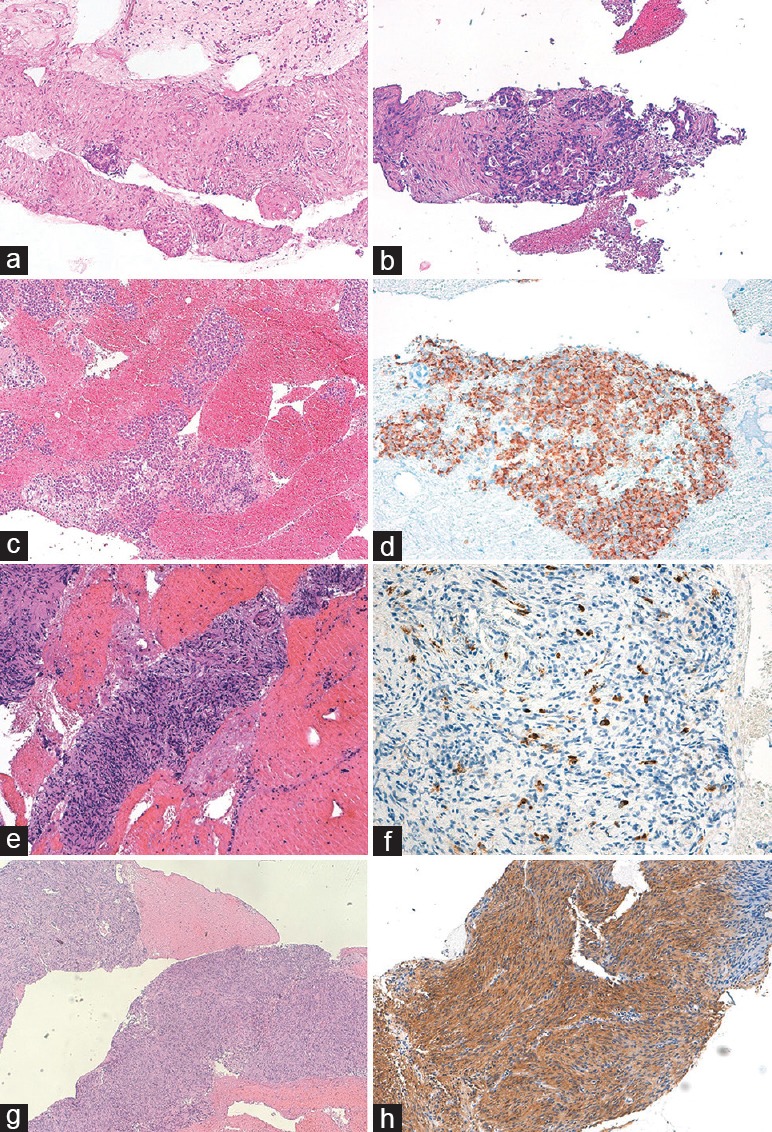
Histological samples from endoscopic ultrasound-guided fine-needle biospy of solid pancreatic lesions. (a) Abundant tissue fragments showing large areas of fibrosis with focal residual endocrine islets suggestive of chronic pancreatitis, (b) infiltration of ductal adenocarcinoma characterized by irregular glands with cribriform architecture, and marked nuclear pleomorphism, (c) groups of monomorphic epithelioid cells positive for chromogranin A at immuhistochemistry, (d) diagnostic for a well-differentiated neuroendocrine neoplasm, (e) abundant tissue fragments with large areas of fibrosis with residual ductal structures and chronic lymphocytic infiltrate, characterized by an elevated number of IgG4 + plasma cells at immunohistochemistry, (f) suggestive for autoimmune pancreatitis, (g) tissue fragments showing solid monomorphic spindle cells proliferation, positive at immunohistochemistry for CD117, and (h) diagnostic for gastrointestinal stromal tumor
Among the 59 lesions, a diagnosis of malignancy was obtained in 55 cases; while in four cases, a histological diagnosis of chronic pancreatitis was made. Subsequent clinical follow-up confirmed a diagnosis of benign condition in all but one patient, in whom pancreatic adenocarcinoma was subsequently diagnosed. Definitive diagnoses were pancreatic adenocarcinoma in 48 (81.4%); neuroendocrine tumor in 5 (8.4%); pancreatic metastasis in 3 (5.1%) from breast cancer in two and colon cancer in one; and chronic pancreatitis in 3 (5.1%). Overall, among the 59 lesions studied, there were 55 true positive, three true negative, and one false negative case. Based on these results, the sensitivity, specificity, negative likelihood ratio, positive likelihood ratio, and diagnostic accuracy were 98.2% (95% confidence interval [CI], 90.6–99.7), 100% (95% CI, 43.6–100), 0.018 (95% CI, 0.003–0.125), 295.6 (95% CI, 0–9.3 × 1010), and 98.3% (95% CI, 94.9–100), respectively.
DISCUSSION
We performed a retrospective study to evaluate the diagnostic performance of the newly available 22-gauge Acquire™ biopsy needle in a cohort of consecutively enrolled patients with pancreatic solid lesions. Overall, procurement of a sample for histological evaluation was achieved in 93.2% of the cases, with a diagnostic accuracy of 98.3%.
In 1992, Vilmann et al.[29] firstly reported the possibility of performing EUS-guided sampling, which in the following years has been mainly done by collecting specimens for cytological examination. However, the widespread dissemination of EUS-FNA worldwide and in the community has been limited by the need for cytological evaluation for two main reasons:[30] (i) The high degree of expertise required for cytology, which is rarely found outside high volume tertiary care centers;[7] (ii) the limited availability of ROSE to immediately assess the collected specimens, which is needed to reach a diagnostic accuracy >90%.[2,3,4,5] As a consequence, EUS-FNA has been perceived for many years to have a limited utility and it has been consequently underutilized.[6]
Different techniques and specifically designed needles to gather tissue core biopsy samples for histological examination have been developed in the last decade to overcome the aforementioned limitations of EUS-FNA.[1] The Tru-Cut biopsy needle (QuickCore®, Cook Medical, Bloomington, IN, USA), the first biopsy needle that was developed, did not show any advantage over conventional FNA needles.[31,32] More recently, a new generation of reverse side-bevel histology needles for EUS-FNB, the Procore™ needles (Cook Medical), has been developed and tested in clinical practice. Unfortunately, they also did not demonstrate any clear superiority over conventional FNA needles.[13,14,15,16,17] On the other hand, there have been reports of a very high capability of procuring samples for histological examination by standard 19-gauge needles, with a very high diagnostic accuracy in patients with various indications.[18,19,20,21,22,23,24,25,26]
Despite the available evidence, because of fear of complications and the difficulty in using large caliber needles, especially in the duodenum, 19-gauge needles have never gained full acceptance among both expert and nonexpert endosonographers. However, in the era of individualized medicine, a shift from EUS-FNA to EUS-FNB is expected to occur.[8,9,10,11,12] Consequently, some authors emphasized the need to develop the proper needle (s), which will be able to acquire enough tissue to perform all the studies required and that will have to meet both the needs of experts and also of practitioners in the community.[12]
In our multicenter retrospective study, we evaluated the performance of a newly developed small caliber needle, the 22-gauge Acquire™ needle, which has been specifically designed to perform tissue core biopsy and overcome the above-described difficulties. Among 59 patients with pancreatic solid lesions, we found that the 22-gauge Acquire™ needle was able to retrieve a tissue core biopsy sample for histological examination in 93.2% of the lesions. Moreover, in the remaining four additional cases (6.8%), a sample for cytological examination was obtained. This procurement yield for a tissue core biopsy specimen of 93.2% is extremely high and is in concordance with the study by Bang et al.[27] in which using the same needle with the cell block technique, a sample adequate for ROSE evaluation and histological diagnosis was found in 96.7% of the patients. The procurement yield is slightly higher to the 88% reported by DiMaio et al.[33] in 147 lesions located throughout the gastrointestinal tract and slightly lower than the 99% reported by Nayar et al.[34] in 101 pancreatic lesions. In both studies, the authors used the newly available 22-gauge and 25-gauge SharkCore™ needles, which are also small caliber EUS-FNB needles, with a modified needle tip.
When combining samples obtained for both histological and cytological analysis, a diagnostic accuracy of 98.3% was found in our study. This value is comparable with the one reported by Nayar et al.[34] in pancreatic lesions only and to the diagnostic accuracy >90% observed when a FNA procedure is performed with ROSE.[2,3,4,5] Unfortunately, our study is not comparative and no conclusions about the value of FNB performed with the 22-gauge Acquire™ as compared to FNA with ROSE can be drawn.
Interestingly, we reported no procedure-related complications, while Bang et al.[27] reported a 3.3% rate of adverse events (one patient who had arterial bleeding that could be managed endoscopically with clip placement). Future studies on a very large patient population with different locations and types of lesions will be needed to address if the design of the tip of this needle can be responsible for an increase rate of complications.
CONCLUSIONS
Our study demonstrates that in patients with solid pancreatic lesions, the 22-gauge Acquire™ needle has very good performance for both procurement of tissue core biopsy for histological examination and diagnostic accuracy. Large multicenter prospective comparative studies are warranted to further evaluate the usefulness of these needles as compared to EUS-FNA with ROSE that represent the actual standard of care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wani S, Muthusamy VR, Komanduri S. EUS-guided tissue acquisition: An evidence-based approach (with videos) Gastrointest Endosc. 2014;80:939–59.e7. doi: 10.1016/j.gie.2014.07.066. [DOI] [PubMed] [Google Scholar]
- 2.Eloubeidi MA, Tamhane A, Jhala N, et al. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: Implications for the endosonographer and patient management. Am J Gastroenterol. 2006;101:2841–7. doi: 10.1111/j.1572-0241.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 3.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 4.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matynia AP, Schmidt RL, Barraza G, et al. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:697–705. doi: 10.1111/jgh.12431. [DOI] [PubMed] [Google Scholar]
- 6.Kalaitzakis E, Panos M, Sadik R, et al. Clinicians’ attitudes towards endoscopic ultrasound: A survey of four European countries. Scand J Gastroenterol. 2009;44:100–7. doi: 10.1080/00365520802495545. [DOI] [PubMed] [Google Scholar]
- 7.Jhala NC, Jhala DN, Chhieng DC, et al. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist's perspective. Am J Clin Pathol. 2003;120:351–67. doi: 10.1309/MFRF-J0XY-JLN8-NVDP. [DOI] [PubMed] [Google Scholar]
- 8.Varadarajulu S, Hawes RH. The changing paradigm in EUS-guided tissue acquisition. Gastrointest Endosc Clin N Am. 2014;24:1–7. doi: 10.1016/j.giec.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Braat H, Bruno M, Kuipers EJ, et al. Pancreatic cancer: Promise for personalised medicine? Cancer Lett. 2012;318:1–8. doi: 10.1016/j.canlet.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 10.Brais RJ, Davies SE, O’Donovan M, et al. Direct histological processing of EUS biopsies enables rapid molecular biomarker analysis for interventional pancreatic cancer trials. Pancreatology. 2012;12:8–15. doi: 10.1016/j.pan.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Kim EY. Fine-needle biopsy: Should this be the first choice in endoscopic ultrasound-guided tissue acquisition? Clin Endosc. 2014;47:425–8. doi: 10.5946/ce.2014.47.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panic N, Larghi A. Techniques for endoscopic ultrasound-guided fine-needle biopsy. Gastrointest Endosc Clin N Am. 2014;24:83–107. doi: 10.1016/j.giec.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Iwashita T, Nakai Y, Samarasena JB, et al. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013;77:909–15. doi: 10.1016/j.gie.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Attili F, Petrone G, Abdulkader I, et al. Accuracy and inter-observer agreement of the Procore™ 25 gauge needle for endoscopic ultrasound-guided tissue core biopsy. Dig Liver Dis. 2015;47:943–9. doi: 10.1016/j.dld.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 16.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: Results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–96. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 17.Iglesias-García J, Abdulkader I, Lariño-Noia J, et al. Evaluation of the adequacy and diagnostic accuracy of the histology samples obtained with a newly designed 19-gauge EUS histology needle. Rev Esp Enferm Dig. 2014;106:6–14. doi: 10.4321/s1130-01082014000100002. [DOI] [PubMed] [Google Scholar]
- 18.Itoi T, Itokawa F, Sofuni A, et al. Puncture of solid pancreatic tumors guided by endoscopic ultrasonography: A pilot study series comparing Trucut and 19-gauge and 22-gauge aspiration needles. Endoscopy. 2005;37:362–6. doi: 10.1055/s-2004-826156. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda I, Tsurumi H, Omar S, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919–24. doi: 10.1055/s-2006-944665. [DOI] [PubMed] [Google Scholar]
- 20.Iwashita T, Yasuda I, Doi S, et al. The yield of endoscopic ultrasound-guided fine needle aspiration for histological diagnosis in patients suspected of stage I sarcoidosis. Endoscopy. 2008;40:400–5. doi: 10.1055/s-2007-995593. [DOI] [PubMed] [Google Scholar]
- 21.Larghi A, Verna EC, Ricci R, et al. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: A prospective study. Gastrointest Endosc. 2011;74:504–10. doi: 10.1016/j.gie.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Larghi A, Capurso G, Carnuccio A, et al. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: A prospective study. Gastrointest Endosc. 2012;76:570–7. doi: 10.1016/j.gie.2012.04.477. [DOI] [PubMed] [Google Scholar]
- 23.Iwashita T, Yasuda I, Doi S, et al. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2012;10:316–22. doi: 10.1016/j.cgh.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Yasuda I, Goto N, Tsurumi H, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymphoproliferative disorders: Feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am J Gastroenterol. 2012;107:397–404. doi: 10.1038/ajg.2011.350. [DOI] [PubMed] [Google Scholar]
- 25.Stavropoulos SN, Im GY, Jlayer Z, et al. High yield of same-session EUS-guided liver biopsy by 19-gauge FNA needle in patients undergoing EUS to exclude biliary obstruction. Gastrointest Endosc. 2012;75:310–8. doi: 10.1016/j.gie.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 26.Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc. 2012;76:336–43. doi: 10.1016/j.gie.2012.04.455. [DOI] [PubMed] [Google Scholar]
- 27.Bang JY, Hebert-Magee S, Hasan MK, et al. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig Endosc. 2017;29:338–46. doi: 10.1111/den.12769. [DOI] [PubMed] [Google Scholar]
- 28.Attam R, Arain MA, Bloechl SJ, et al. “Wet suction technique (WEST)”: A novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401–7. doi: 10.1016/j.gie.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 30.Larghi A, Eguia V, Hassan C, et al. Economic crisis: The right time to widen endoscopic ultrasound utilization. Endoscopy. 2014;46:80–1. doi: 10.1055/s-0033-1344858. [DOI] [PubMed] [Google Scholar]
- 31.Thomas T, Kaye PV, Ragunath K, et al. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: A large tertiary referral center experience. Am J Gastroenterol. 2009;104:584–91. doi: 10.1038/ajg.2008.97. [DOI] [PubMed] [Google Scholar]
- 32.DeWitt J, Cho CM, Lin J, et al. Comparison of EUS-guided tissue acquisition using two different 19-gauge core biopsy needles: A multicenter, prospective, randomized, and blinded study. Endosc Int Open. 2015;3:E471–8. doi: 10.1055/s-0034-1392222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiMaio CJ, Kolb JM, Benias PC, et al. Initial experience with a novel EUS-guided core biopsy needle (SharkCore): Results of a large North American multicenter study. Endosc Int Open. 2016;4:E974–9. doi: 10.1055/s-0042-112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nayar MK, Paranandi B, Dawwas MF, et al. Comparison of the diagnostic performance of 2 core biopsy needles for EUS-guided tissue acquisition from solid pancreatic lesions. Gastrointest Endosc. 2017;85:1017–24. doi: 10.1016/j.gie.2016.08.048. [DOI] [PubMed] [Google Scholar]


