Abstract
Little is known about dysphagia after pontine infarction. In this study, we evaluated the incidence of dysphagia after isolated pontine infarction and identified the predictive factors for the occurrence of dysphagia. A total of 146 patients were included in this study. All patients underwent clinical testing for dysphagia within 1 day after admission and at the time of discharge. We compared the incidence of dysphagia between patients with unilateral pontine infarction and those with bilateral pontine infarction. To evaluate the functional status of patients, we investigated their initial modified Rankin Scale (mRS) score and initial National Institutes of Health Stroke Scale (NIHSS) score within 1 day of admission. Of 146 patients, 50 (34.2%) had dysphagia initially within 1 day after admission. At the second evaluation at the time of discharge, dysphagia was diagnosed in 24 patients (16.4%). Patients with bilateral pontine infarction were more likely to present with dysphagia. In addition, clinical severity (in terms of mRS and NIHSS scores) was identified as a predictor of dysphagia in patients with cerebral infarction (multiple binary logistic regression analysis, mRS: P = 0.011, NIHSS: P = 0.004). Dysphagia frequently occurs in patients with isolated pontine infarction. Clinicians should pay particular attention to the occurrence of dysphagia, especially in patients with bilateral pontine infarction or high functional disability.
Keywords: dysphagia, pons, infarction, stroke, prediction, NIHSS score, MRS score
Introduction
Dysphagia is a frequent consequence of stroke, with an estimated incidence ranging from 29% to 81% (Barer, 1989; Martino et al., 2005; Flowers et al., 2011). Dysphagia after stroke not only increases morbidity and mortality but also significantly affects the quality of life (Martino et al., 2005). Prompt evaluation and treatment of dysphagia can mitigate the development of secondary complications and allow early reintegration of the patient into society (Gonzalez-Fernandez et al., 2013).
Pontine infarction constitutes approximately 7% of all ischemic infarctions and 15% of acute vertebrobasilar ischemic strokes, and these incidences are higher than those of any other isolated infarcts of the brainstem (Silverstein, 1964; Saia and Pantoni, 2009). Although some previous studies have evaluated dysphagia after pontine infarction, most previous studies on pontine infarction focused on other clinical features, such as motor and cognitive functions (Bassetti et al., 1996; Kumral et al., 2002; Kunz et al., 2003).
In this study, we investigated the incidence of dysphagia after isolated pontine infarction. Furthermore, we evaluated the predictive factors for the occurrence of dysphagia.
Subjects and Methods
Subjects
The Asan Medical Center Institutional Review Board (No. 2014-1072) of a university hospital approved the study protocol. A total of 146 patients admitted to Department of Rehabilitation Medicine of Asan Medical Center between January 2009 and June 2014 were enrolled in this retrospective study according to the following criteria: (1) pure pontine infarction without other lesions; (2) first occurrence of stroke; (3) age 20–89 years; and (4) no other causes of dysphagia, such as pharyngolaryngeal cancer, from onset to final evaluation. All recruited patients underwent initial treatment for acute cerebral infarct at the Department of Neurology of a university hospital and were then either transferred to the Department of Physical Medicine and Rehabilitation of the same hospital or discharged. All patients were diagnosed as having pure pontine infarction based on magnetic resonance imaging findings. The diagnosis was confirmed by a neurologist and a radiologist. The patients were subdivided on the basis of the main vascular territory and topographic localization according to previously published anatomical studies (Gillilan, 1964; Tatu et al., 2001). Furthermore, we classified patients as exhibiting either unilateral or bilateral lesions. Data on patient age, sex, hospital length of stay, and presence of dysarthria and dysphagia at admission were collected.
Assessments
All patients underwent the standardized clinical bedside test for dysphagia within 1 day of admission. The tests were conducted in three steps according to previous studies (Gillilan, 1964; Tatu et al., 2001). The first step served to identify the level of consciousness and collaboration capacity of the patient (drowsy mental status, failure to follow simple commands, not opening the mouth, dysarthria, facial palsy, impairment of the tongue, or dysphonia). The second step consisted of the dry swallowing (saliva) test. The third step involved swallowing 5 mL of water with concomitant pulse oximetry while carefully observing for signs of oral-phase dysphagia (spillage of water from the lips, delay in swallowing, or abnormality or absence of tongue movement) or signs of penetration/aspiration (wet or gurgling voice, coughing, or < 90% oxygen saturation in pulse oximetry). On the basis of this three-step bedside swallowing assessment, each patient was classified as either dysphagic (abnormality of at least one of the assessment criteria in each step) or non-dysphagic. We also performed the test for dysphagia at the time of discharge.
To evaluate the functional status of patients, we investigated their initial modified Rankin Scale (mRS) score (Quinn et al., 2009) and initial National Institutes of Health Stroke Scale (NIHSS) score (Heldner et al., 2013) within 1 day of admission. A higher score on the mRS means greater disability or dependence in daily activities: scores range from 0 (no symptoms at all) to 6 (death). The NIHSS is a graded neurological examination assessing consciousness, eye movements, visual fields, motor and sensory impairment, ataxia, speech, cognition, and inattention. The NIHSS scores range from 0 to 42. More points are given for greater deficiencies.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS ver. 23.0; IBM, Armonk, NY, USA). Pearson's chi-square test, Fisher's exact test, and Mann-Whitney U tests were used to compare demographic data, clinical data, and incidence of dysphagia between the unilateral and bilateral pontine infarction groups. Two-tailed tests were performed. A P value of < 0.05 was considered statistically significant for all tests. For univariate analysis, chi-square test was used to determine association factors with dysphagia within 1 day after admission. For multivariate analysis, multiple binary logistic regression was used and statistically significant variables in univariate analysis were used as covariate variables.
Results
Infarction territory and dysphagia incidence
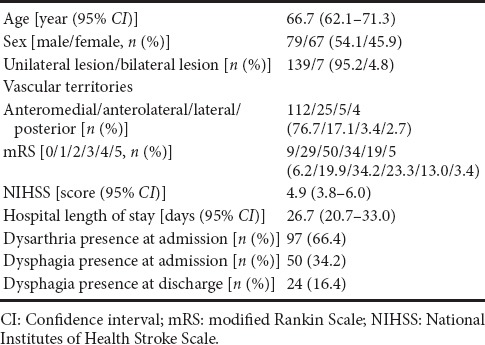
A total of 146 patients (79 men and 67 women; mean age, 66.7 years) with isolated pontine infarction were included in this study (Table 1). Anteromedial territory infarct was present in 112 patients (77%), anterolateral territory infarct in 25 patients (17%), lateral territory infarct in 5 patients (3%), and posterior territory infarct in 4 patients (3%). A total of 139 (95%) patients had unilateral pontine infarction and 7 (5%) had bilateral pontine infarction. Of 146 patients, 50 (34.2%) had dysphagia within 1 day after admission. At the second evaluation at the time of discharge, dysphagia was diagnosed in 24 patients (16.4%).
Table 1.
Demographic data and clinical characteristics of patients with isolated pontine infarction
Predictive factors for dysphagia
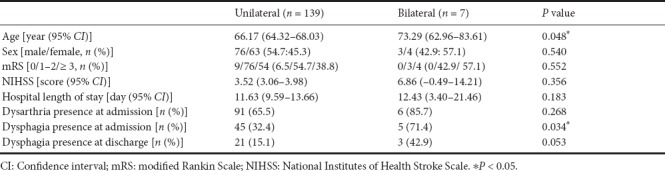
Patients with bilateral pontine lesions were older than those with unilateral pontine lesions (P = 0.048) (Table 2). Although there were no significant differences in the sex ratio, mRS score, NIHSS score, and hospital length of stay between the unilateral and bilateral pontine infarction groups, a greater proportion of patients with bilateral pontine infarction had dysphagia at admission than patients with unilateral lesions (P = 0.034). Moreover, there was a tendency of a higher incidence of dysphagia in patients with bilateral pontine infarction at the time of discharge, although the difference was not significant (P = 0.053).
Table 2.
Comparison of demographic and clinical characteristics between unilateral and bilateral pontine infarct patients
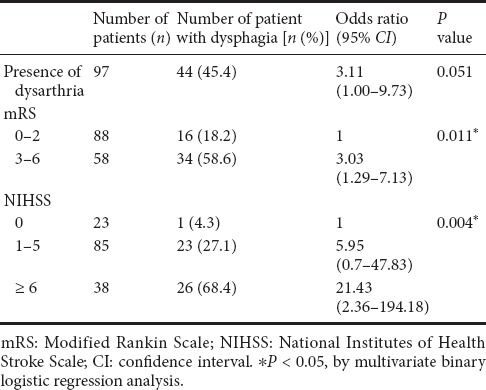
In the univariate analysis, NIHSS score, mRS score, and presence of dysarthria were significantly associated with the development of dysphagia (P < 0.001) (Table 3). Bilateral lesion was a marginally significant factor related to dysphagia (P = 0.053). In the multivariate analysis (Table 4), NIHSS and mRS scores were identified as the strongest independent predictors of dysphagia development in patients with pontine infarction (P = 0.011 and P = 0.004, respectively).
Table 3.
Univariate analysis of parameters related to dysphagia in patients with isolated pontine infarction
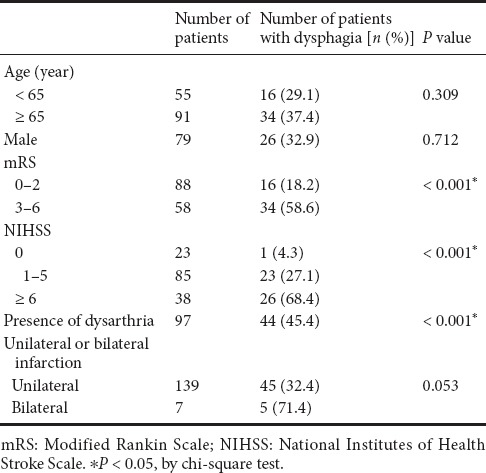
Table 4.
Stepwise multiple regression of parameters related to dysphagia in patients with isolated pontine infarction
Discussion
In the current study, we investigated the incidence of dysphagia and the predictive factors for its occurrence in patients with isolated pontine infarction.
Of 146 recruited patients with isolated pontine infarction, 34.2% developed dysphagia. Furthermore, 16.4% showed dysphagia at an average of about 4 weeks after the onset of the infarct. To date, four studies have investigated the incidence of dysphagia at the acute stage after pontine infarction (Kim et al., 1995; Schmahmann et al., 2004; Maeshima et al., 2012; Lapa et al., 2017). Kim et al. (1995) reported that 13 of their 32 patients (40.6%) had dysphagia. Schmahmann et al. (2004) reported that 10 of their 15 patients (66.7%) showed dysphagia. Maeshima et al. (2012) evaluated the occurrence of dysphagia and reported that 49 of their 68 patients (72.1%) had dysphagia symptoms. Lapa et al. (2017) found that 14 of their 59 patients (23.7%) presented with dysphagia. In the above-mentioned studies, the occurrence of dysphagia varied from 23.7% to 72.1%. These heterogeneous results could be ascribed to the different assessment tools and the small number of recruited subjects. We used standardized clinical tests for dysphagia. Moreover, our study included a larger number of patients than that of previous studies. Regarding dysphagia at the time of discharge after finishing the initial treatment, the above-cited study by Maeshima et al. (2012) observed that 22 patients (32.4%) were receiving a dysphagia diet and 22 patients (32.4%) were receiving enteral feeding at an average of 24.4 days after the onset of pontine infarction. However, unlike us, Maeshima et al. (2012) did not perform clinical tests for the presence of dysphagia. We believe that this is the first study to report dysphagia data at the time of discharge after the initial treatment in patients with pontine infarction.
Additionally, we found that patients with bilateral pontine infarction were more likely to present with dysphagia. In contrast to the medulla oblongata and supratentorial regions, the pons is not considered to contain a swallowing center (Suntrup et al., 2015). In cases of pontine stroke, it is known that the affected corticobulbar tract leads to impairment in swallowing function (Lapa et al., 2017). The corticobulbar tract innervates muscles related to swallowing function unilaterally (Gazzaniga and Smylie, 1990). Thus, bilateral pontine infarction damages the corticobulbar tracts bilaterally, which causes dysfunction of both sides of the swallowing muscles. As a result, in our study, a significantly larger number of patients with bilateral pontine infarction appeared to have developed dysphagia than those with unilateral infarction. To the best of our knowledge, this is the first study to report a difference in the occurrence of dysphagia between patients with unilateral pontine infarction and those with bilateral pontine infarction.
Patients with pontine infarction with high mRS and NIHSS scores in our study had a higher risk of developing dysphagia. This result corroborates with the results of previous studies showing that clinical severity was a predictor of dysphagia in patients with cerebral infarction (Crary et al., 2006; Lapa et al., 2017).
In conclusion, we investigated dysphagia in a relatively large number of patients with isolated pontine infarction. Dysphagia was observed in 34.2% and 16.4% of recruited patients at the acute stage and at the time of discharge, respectively. Bilateral pontine infarction caused dysphagia more frequently than unilateral pontine infarction. Furthermore, higher NIHSS and mRS scores predicted a higher risk of dysphagia development. Therefore, we recommend paying close attention to early swallowing problems, especially when patients have bilateral pontine infarction and show high functional disability.
Our present study has several limitations. First, this study was conducted retrospectively. Second, the number of patients with bilateral pontine infarction (n = 7) was too small to determine clinical significance. Third, we did not investigate the long-term outcome of dysphagia. Fourth, we did not assess the severity of dysphagia with the videofluoroscopic swallowing study. Lastly, we did not evaluate the effect of infarct size or location on the occurrence of dysphagia. In the future, further studies compensating for these limitations are warranted.
Additional file: Open peer review report 1 (165.1KB, pdf) .
Footnotes
Conflicts of interest: There are no conflicts of interest associated with this study.
Financial support: None.
Institutional review board statement: The Asan Medical Center Institutional Review Board (No. 2014-1072) of a university hospital approved the study protocol.
Reporting statement: This study followed the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals developed by the International Committee of Medical Journal Editors.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of Yeungnam University, Republic of Korea.Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: For data sharing, individual participant data will not be available. However, the study protocol and informed consent form will be made available beginning 3 months and ending 5 years following article publication to investigators whose proposed use of the data has been approved by an independent review committee identified to achieve aims in the approved proposal. In order to gain access, data requestors will need to sign a data access agreement. Proposals should be directed to mhchun0@gmail.com
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Heng-Li Tian, Shanghai Sixth People's Hospital, Shanghai Jiaotong University, China.
P-Reviewer: Tian HL; C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Liu XL
References
- 1.Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. J Neurol Neurosurg Psychiatry. 1989;52:236–241. doi: 10.1136/jnnp.52.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassetti C, Bogousslavsky J, Barth A, Regli F. Isolated infarcts of the pons. Neurology. 1996;46:165–175. doi: 10.1212/wnl.46.1.165. [DOI] [PubMed] [Google Scholar]
- 3.Crary MA, Carnaby-Mann GD, Miller L, Antonios N, Silliman S. Dysphagia and nutritional status at the time of hospital admission for ischemic stroke. J Stroke Cerebrovasc Dis. 2006;15:164–171. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Daniels SK, Schroeder MF, McClain M, Corey DM, Rosenbek JC, Foundas AL. Dysphagia in stroke: development of a standard method to examine swallowing recovery. J Rehabil Res Dev. 2006;43:347–356. doi: 10.1682/jrrd.2005.01.0024. [DOI] [PubMed] [Google Scholar]
- 5.Falsetti P, Acciai C, Palilla R, Bosi M, Carpinteri F, Zingarelli A, Pedace C, Lenzi L. Oropharyngeal dysphagia after stroke: incidence, diagnosis, and clinical predictors in patients admitted to a neurorehabilitation unit. J Stroke Cerebrovasc Dis. 2009;18:329–335. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Flowers HL, Skoretz SA, Streiner DL, Silver FL, Martino R. MRI-based neuroanatomical predictors of dysphagia after acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis. 2011;32:1–10. doi: 10.1159/000324940. [DOI] [PubMed] [Google Scholar]
- 7.Gazzaniga MS, Smylie CS. Hemispheric mechanisms controlling voluntary and spontaneous facial expressions. J Cogn Neurosci. 1990;2:239–245. doi: 10.1162/jocn.1990.2.3.239. [DOI] [PubMed] [Google Scholar]
- 8.Gillilan LA. The correlation of the blood supply to the human brain stem with clinical brain stem lesions. J Neuropathol Exp Neurol. 1964;23:78–108. doi: 10.1097/00005072-196401000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Fernandez M, Ottenstein L, Atanelov L, Christian AB. Dysphagia after stroke: an overview. Curr Phys Med Rehabil Rep. 2013;1:187–196. doi: 10.1007/s40141-013-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heldner MR, Zubler C, Mattle HP, Schroth G, Weck A, Mono ML, Gralla J, Jung S, El-Koussy M, Lüdi R, Yan X, Arnold M, Ozdoba C, Mordasini P, Fischer U. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44:1153–1157. doi: 10.1161/STROKEAHA.111.000604. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Lee JH, Im JH, Lee MC. Syndromes of pontine base infarction: a clinical-radiological correlation study. Stroke. 1995;26:950–955. doi: 10.1161/01.str.26.6.950. [DOI] [PubMed] [Google Scholar]
- 12.Kumral E, Bayulkem G, Evyapan D. Clinical spectrum of pontine infarction. Clinical-MRI correlations. J Neurol. 2002;249:1659–1670. doi: 10.1007/s00415-002-0879-x. [DOI] [PubMed] [Google Scholar]
- 13.Kunz S, Griese H, Busse O. Etiology and long-term prognosis of unilateral paramedian pontine infarction with progressive symptoms. Eur Neurol. 2003;50:136–140. doi: 10.1159/000073053. [DOI] [PubMed] [Google Scholar]
- 14.Lapa S, Luger S, Pfeilschifter W, Henke C, Wagner M, Foerch C. Predictors of dysphagia in acute pontine infarction. Stroke. 2017;48:1397–1399. doi: 10.1161/STROKEAHA.116.015045. [DOI] [PubMed] [Google Scholar]
- 15.Maeshima S, Osawa A, Miyazaki Y, Takeda H, Tanahashi N. Functional outcome in patients with pontine infarction after acute rehabilitation. Neurol Sci. 2012;33:759–764. doi: 10.1007/s10072-011-0812-0. [DOI] [PubMed] [Google Scholar]
- 16.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 17.Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin Scale: a systematic review. Stroke. 2009;40:3393–3395. doi: 10.1161/STROKEAHA.109.557256. [DOI] [PubMed] [Google Scholar]
- 18.Saia V, Pantoni L. Progressive stroke in pontine infarction. Acta Neurol Scand. 2009;120:213–215. doi: 10.1111/j.1600-0404.2009.01161.x. [DOI] [PubMed] [Google Scholar]
- 19.Schmahmann JD, Ko R, MacMore J. The human basis pontis: motor syndromes and topographic organization. Brain. 2004;127:1269–1291. doi: 10.1093/brain/awh138. [DOI] [PubMed] [Google Scholar]
- 20.Silverstein A. Acute infarctions of the brain stem in the distribution of the basilar artery. Confin Neurol. 1964;24:37–61. [PubMed] [Google Scholar]
- 21.Suntrup S, Kemmling A, Warnecke T, Hamacher C, Oelenberg S, Niederstadt T, Heindel W, Wiendl H, Dziewas R. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: dysphagia incidence, severeity and aspiration. Eur J Neurol. 2015;22:832–838. doi: 10.1111/ene.12670. [DOI] [PubMed] [Google Scholar]
- 22.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of human brain. In: Bogousslavsky J, Caplan LR, editors. Stroke Syndromes. 2nd ed. Cambridge: Cambridge University Press; 2001. pp. 375–404. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


