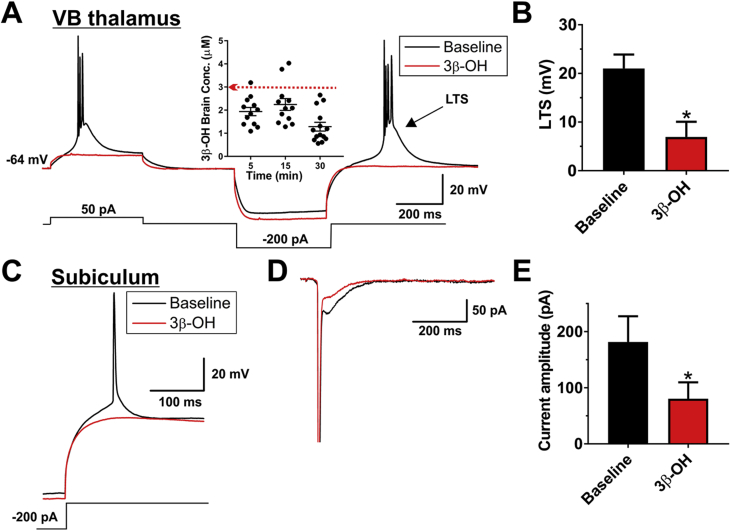
Fig 4.
The effects of (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) on excitability of thalamic and subicular neurones. (A) Original traces from a representative thalamic neurone in control pre-drug conditions (black trace) portraying the loss of burst firing pattern as a response to both depolarising and hyperpolarising stimulus and in the presence of 3 μM 3β-OH (red trace). The inset shows concentrations of 3β-OH in rat brain achieved 5, 15, and 30 min after i.p. injection of 10 mg kg−1 3β-OH. (B) Application of 3 μM 3β-OH also significantly decreased the average rebound low-threshold spike (LTS), which underlies bursting in these neurones (paired t-test: t4=3.14, P=0.035; n=5 neurones, three rats). (C) Original traces from a representative subicular neurone depicting control (black trace) and the effect of 3 μM 3β-OH (red trace) on the rebound firing pattern to a 200 pA hyperpolarising stimulus. On average 3 μM 3β-OH decreased the number of rebound action potentials from 1.41 (0.26) to 0.76 (0.21) (paired t-test: t(5)=3.81, P=0.013; n=6 neurones, three rats). (D) Original traces from a representative subicular neurone showing that 3β-OH reduced the amplitude of inward calcium currents (evoked using Vh of −90 mV and Vt of −40 mV). (E) Bar graphs showing averages from multiple experiments similar to panel (D) of this figure, which demonstrate that 3β-OH reduced the amplitude of calcium current by approximately 50% compared with baseline (pre-drug) control in the same cells (paired t-test: t3=4.30, P=0.023; n=4 neurones, one rat), *P<0.05 vs baseline pre-drug conditions.