Abstract
Neuraxial clonidine improves postoperative analgesia in the general surgical population. The efficacy and safety of neuraxial clonidine as a postoperative analgesic adjunct in the Caesarean section population still remains unclear. This systematic review and meta-analysis aims to evaluate the effect of perioperative neuraxial clonidine on postoperative analgesia in women having Caesarean section under neuraxial anaesthesia. We included randomized controlled trials comparing the analgesic efficacy of the perioperative administration of neuraxial clonidine alone or in combination with a local anaesthetic and/or opioids in women having elective Caesarean section under neuraxial anaesthesia when compared with placebo. PubMed, the Cochrane Central Register of Controlled Trials, and EMBASE were searched until February 2017. Eighteen studies were included in the meta-analysis. Neuraxial clonidine reduced 24 h morphine consumption [mean difference (MD): −7.2 mg; 95% confidence interval (CI): −11.4, −3.0 mg; seven studies] and prolonged time to first analgesic request (MD: 135 min; 95% CI: 102, 168 min; 16 studies) when compared with the control group. Neuraxial clonidine increased intraoperative hypotension [odds ratio (OR): 2.849; 95% CI: 1.363, 5.957], intraoperative sedation (OR: 2.355; 95% CI: 1.016, 5.459), but reduced the need for intraoperative analgesic supplementation (OR: 0.224; 95% CI: 0.076, 0.663). The effect of clonidine on intraoperative bradycardia, intraoperative and postoperative nausea and vomiting, postoperative sedation, and pruritus were inconclusive. Neuraxial clonidine did not negatively impact neonatal umbilical artery pH or Apgar scores. This review demonstrates that neuraxial clonidine enhances postoperative analgesia in women having Caesarean section with neuraxial anaesthesia, but this has to be balanced against increased maternal adverse effects.
Keywords: adrenergic alpha-2 receptor agonist, caesarean section, clonidine
Caesarean section is one of the most common surgical procedures performed in the obstetric patient population.1 Pregnant women rate pain during and after Caesarean delivery as their primary concern.2 The postoperative management of pain after Caesarean section still remains a challenge. Poorly controlled acute postoperative pain can affect a new mother's mobility, mood, and ultimately her ability to care for her newborn baby.3 Poorly controlled acute postoperative pain also increases the risk for persistent pain for up to 8 weeks postpartum.3 Current strategies for the management of postoperative pain mainly involve the use of neuraxial opioids when neuraxial anaesthetic techniques are used. Even though neuraxial opioids have improved the quality of postoperative analgesia, they are associated with opioid related side effects such as nausea, vomiting, and pruritus.4 Additionally, in an increasing number of opioid tolerant patients, opioids may be less effective.5 Furthermore, in some countries long-acting opioids such as preservative-free morphine or diamorphine may not be readily available. As a result, there is renewed interest in the use of non-opioid analgesic adjuncts administered via the neuraxial route, such as clonidine, for the optimisation of postoperative pain after Caesarean section.
Clonidine is an α2 agonist that mediates its analgesic effect via the α2 receptor located post-synaptically on the dorsal horn of the spinal cord. Stimulation of the α2 receptor reduces afferent transmission of pain producing analgesia.6 In the general surgical population, the administration of i.v. clonidine to patients receiving general anaesthesia reduced morphine consumption and pain scores at 24 h after surgery, when compared with placebo.7 Similarly, the administration of clonidine intrathecally enhanced the effect of local anaesthetics and opioids resulting in a longer time to first request for analgesia and a reduction in 24 h morphine consumption.8, 9 The analgesic effect of neuraxial clonidine for post-Caesarean analgesia still remains unclear, with studies investigating its analgesic effect yielding conflicting results. Recent evidence also suggests that clonidine may reduce acute hyperalgesia and possibly the development of chronic persistent pain after Caesarean section.10 However, while clonidine may improve post-Caesarean delivery analgesia, it has been associated with an increased incidence of maternal hypotension, sedation, and foetal acidosis, limiting its clinical use.8, 11
To address these concerns we performed a systematic review and meta-analysis to evaluate the effect of perioperative neuraxial clonidine administration on postoperative analgesia in women having Caesarean section under neuraxial anaesthesia. Our hypothesis was that in women having Caesarean section under neuraxial anaesthesia, the administration of neuraxial clonidine would improve postoperative analgesia. This improvement would be determined by a reduction in morphine consumption and/or an increase in the time to first analgesic request, our primary outcomes of interest. We also investigated whether the administration of clonidine would be associated with a reduction in maternal opioid-related side effects. Finally, we investigated whether the administration of clonidine would be associated with an increase in maternal or foetal adverse effects.
Methods
This systematic review and meta-analysis was reported in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement.12
Eligibility criteria
We performed a search of the published literature for randomised controlled trials comparing the analgesic efficacy of the perioperative administration of single or multiple doses of neuraxial clonidine alone or in combination with a local anaesthetic and/or opioid in women having elective Caesarean section under neuraxial anaesthesia when compared with placebo. Specifically, these trials needed to report 24 h opioid analgesic consumption (or the closest time point) and/or time to first analgesic request in both experimental arms. When studies reported multiple treatment arms using additional non-narcotic adjuncts, only data from the groups utilizing an amide local anaesthetic (with/without opioid) and clonidine (with/without opioid) were extracted. We included studies where neuraxial clonidine was administered in addition to short and long acting neuraxial opioids for surgical anaesthesia and/or postoperative analgesia. However, we excluded studies where clonidine was co-administered with differing doses of opioids for determining synergism or relative potency. We also excluded studies where the dose of local anaesthetic was different in the control and treatment arms of the study and where neuraxial clonidine was administered in patients who received general anaesthesia. Data from abstracts and unpublished trials were excluded. Eligibility was assessed independently by two individuals (T.K.A. and B.M.M.). Disagreements were reconciled by discussion and then by a 3rd member of the study team (A.S.H.) when necessary.
Search strategy
We searched PubMed (1966–2017), the Cochrane Central Register of Controlled Trials, and EMBASE using the search strategies described in the supplementary file up to February 2017. We imposed no language restrictions. The bibliographies of retrieved trials were also used to identify other relevant articles. Where appropriate, authors were contacted for missing or additional data. The methodological quality of included studies was assessed by two persons (T.K.A. and R.Y.K.) using the Cochrane collaboration tool for assessing risk of bias. Included studies were assessed for selection bias, performance bias, detection bias, attrition bias, and reporting bias.13 Studies were assessed as low risk of bias (low risk of bias for all key domains), unclear risk of bias (unclear risk of bias for any of the key domains), or high risk of bias (high risk of bias for one or more key domains).13
Data were extracted and entered in a Microsoft Excel® (Microsoft Corporation, WA, USA) spreadsheet independently by two authors (T.K.A. and B.M.M.) and checked for accuracy by a third author (R.Y.K.). We extracted data on the country where the study was performed, neuraxial anaesthetic technique, type and dose of local anaesthetic administered, type and dose of neuraxial opioid administered, timing of administration and route of administration (spinal vs epidural) of neuraxial clonidine, and postoperative analgesic regime. We also extracted data on:
-
1.
Our primary outcomes: i.v. morphine consumption at 24 h (or closest reported time point) and the time to first analgesic request. When studies reported postoperative analgesic consumption using other opioids or anti-inflammatory agents, they were converted to i.v. morphine equivalents using the following conversion factors: i.v. ketorolac 30 mg was equivalent to 10 mg of i.v. morphine and 100 mg of meperidine was equivalent to 10 mg of i.v. morphine.14, 15
-
2.
Other analgesic outcomes: intraoperative need for supplemental analgesia, postoperative pain scores on movement at 0–6 and 6–24 h.
-
3.
Maternal adverse effects: intraoperative hypotension, intraoperative vasopressor dose requirements, intraoperative bradycardia, intra- and postoperative nausea and vomiting, intra- and postoperative sedation, pruritus, and respiratory depression. Vasopressor doses were converted to an equivalent dose of ephedrine when phenylephrine was the vasopressor used based on a potency ratio of 81.2 between phenylephrine and ephedrine.16
-
4.
Neonatal outcomes: foetal umbilical artery pH and Apgar scores at 1 and 5 min.
Authors were contacted to provide additional data that were not reported in the manuscript or that were presented graphically. Alternately, we also extracted data on outcomes from graphical information using the software GraphClick (Version 3.0.3, Arizona Software, www.arizona-software.ch/graphclick) when the raw data were not available from authors.
Data analysis
Data from dichotomous outcomes were summarized using odds ratio (OR) and 95% confidence intervals (CI). The number needed to treat (NNT) and number needed to harm (NNH) were computed for statistically significant outcomes. Continuous outcomes extracted as mean and standard deviation were summarized as mean difference (MD) and 95% CI. Where appropriate, when data were expressed as median, inter-quartile range and range, they were converted to means and standard deviation.17 In studies investigating multiple doses of clonidine, treatment groups were combined to allow a single pairwise comparison with the control group. A random effects statistical model was used as the default for the analysis. Forest plots were used to graphically represent and evaluate treatment effects. Statistical heterogeneity was formally assessed using the I2 test (I2>50% defined as significant heterogeneity). To test the validity of our results we performed a sensitivity analysis for the primary outcomes after excluding studies with a high risk of bias. To explore the causes of heterogeneity on our primary outcomes, we planned a priori to perform subgroup analyses using data from studies where clonidine was administered exclusively by the intrathecal route or epidural route. We also performed a subgroup analysis on studies where clonidine was co-administered without long acting neuraxial opioids such as morphine. Subgroup analyses were only performed when three or more studies met the criteria for inclusion. To determine the effect of dose on our primary outcomes, we compared studies and/or subgroups where clonidine was administered at a dose ≤75 μg (or 1 μg kg−1) with those where clonidine was administered at a dose of >75 μg (or 1 μg kg−1) using the Q-test for heterogeneity. Publication bias for the primary outcomes was initially assessed using funnel plots and the regression test described by Egger and colleagues.18 When there was evidence of funnel plot asymmetry, we attempted to investigate the cause of this asymmetry by examining contour-enhanced funnel plots19 and determining the location and significance of any missing studies using the trim and fill method.20 Analyses were performed using Comprehensive Meta-Analysis (Version 2.2.050, Biostat™, Englewood, NJ, USA) and the metafor package in R version 3.1.1 (R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/). A P value <0.05 was considered to indicate statistical significance.
Results
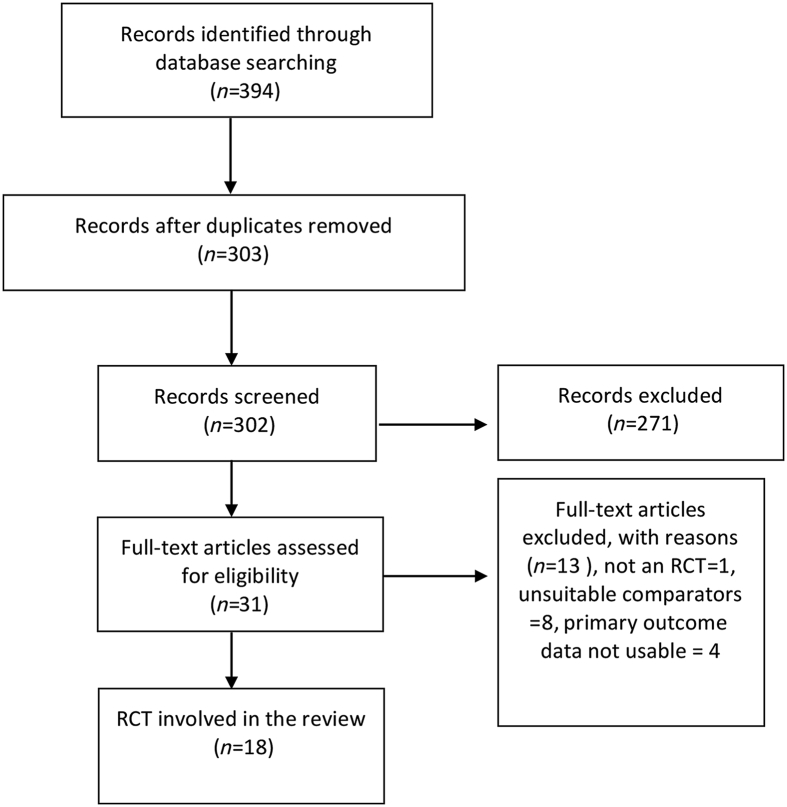
Our search returned 394 articles initially. Of these, 31 studies were identified for full review (Fig. 1). We then excluded a further 13 studies, leaving 18 studies10, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 for inclusion and analysis. Three authors provided additional data on request.10, 29, 33 The characteristics of the included studies are shown in Table 1. The risk of bias summary for each study is summarized in Table 2. Clonidine was administered at doses ranging from 30 to 800 μg. Clonidine was administered via the intrathecal route in 12 studies10, 21, 22, 24, 29, 30, 31, 32, 33, 35, 36, 37 and via the epidural route in the six remaining studies.23, 25, 28, 34 In two of the epidural studies, clonidine was administered as a bolus followed by a continuous epidural infusion.25, 27 In the remaining four studies,23, 26, 28, 34 clonidine was administered as a bolus, with two studies administering repeated epidural boluses.23, 34 Clonidine was administered with bupivacaine in 14 studies,10, 21, 22, 23, 24, 28, 29, 30, 31, 32, 33, 35, 36, 37 with ropivacaine in one study34 and without local anaesthetic or opioid in three studies.25, 26, 27 Clonidine was administered as part of the neuraxial anaesthetic technique in 13 studies10, 21, 22, 24, 28, 29, 30, 31, 32, 33, 35, 36, 37 but administered at the end of surgery in four studies.23, 25, 26, 27 In the study by Huntoon and colleagues,25 clonidine was administered at the end of surgery after epidural anaesthesia with bupivacaine or 2-chlorprocaine. As prior administration of 2-chlorprocaine may inhibit the effects of subsequently administered epidural analgesic agents,25, 38 we extracted data from the patients receiving epidural bupivacaine only. In one study, clonidine was administered as part of the anaesthetic technique for Caesarean section at one dose and administered at the end of surgery at a different dose.34 For this study, we only extracted data on intraoperative outcomes and time to first analgesic request.
Fig 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram. RCT = Randomised Controlled Trial.
Table 1.
Study characteristics of studies included in the review. CSE, combined spinal epidural; MAP, mean arterial pressure; PCA, patient controlled analgesia; SBP, systolic blood pressure; VAS, visual analogue pain scores.
| Study ID | Country of origin | Anaesthetic technique | Local anaesthetic used for anaesthesia | Neuraxial route of clonidine administration | Dose of clonidine | Control group (n) | Intervention group (n) | Timing of clonidine administration | Postoperative analgesic regime | Definition of intraoperative hypotension |
|---|---|---|---|---|---|---|---|---|---|---|
| Lavand'homme and colleagues10 | Belgium | Spinal | Hyperbaric bupivacaine dose was adjusted based on patient height: 9 mg when <160 cm, 10 mg for height between 160 and 175 cm, and 11 mg for >175 cm | Intrathecal | 75,150 μg | Hyperbaric bupivacaine + sufentanil 2 μg (n = 32) | Group 1: hyperbaric bupivacaine + sufentanil 2 μg + clonidine 75 μg (n=32) Group 2: hyperbaric bupivacaine + clonidine 150 μg (n=32) | At spinal anaesthesia | IV morphine PCA analgesia All the parturients received i.v. postoperative diclofenac 150 mg daily (started in the recovery room) + i.v. acetaminophen 1 g 6 h−1 as needed | 20% Reduction from the pre-anaesthetic baseline SBP |
| van Tuijl and colleagues33 | The Netherlands | Spinal | Hyperbaric bupivacaine 11 mg | Intrathecal | 75 μg | Hyper baric bupivacaine 0.5% (2.2 ml) + 0.5 ml saline 0.9% (total 2.7 ml) (n=53) | Hyperbaric bupivacaine 0.5 (2.2 ml) + clonidine 75 μg in 0.5 ml saline 0.9% (total 2.7 ml) (n=53) | At spinal anaesthesia | IV PCA morphine + i.v. bolus morphine (5 mg) if VAS >4 and repeated once if VAS did not decrease below 4 within 20 min | 20% Reduction from the baseline MAP |
| Paech and colleagues29 | Australia | Spinal | Hyperbaric bupivacaine 12.5 mg | Intrathecal | 30, 60, 90, 150 μg | Hyperbaric 0.5% bupivacaine 2.5 ml + morphine 100 μg (n=39) | Hyperbaric 0.5% bupivacaine 2.5 ml + morphine 100 μg in all groups plus Group1: clonidine 30 μg (n=41) Group 2: clonidine 60 μg (n=38) Group 3: clonidine 90 μg (n=38) Group 4: clonidine 150 μg (n=37). | At spinal anaesthesia | IV PCA morphine, naproxen 500 mg (rectally) at end of surgery and then 500 mg orally twice per day | 20% reduction in baseline SBP |
| Benhamou and colleagues21 | France | Spinal | Hyperbaric bupivacaine 0.6 mg/cm of body height | Intrathecal | 75 μg | Hyperbaric bupivacaine and 1 ml of saline (n=26) | Group 1: hyperbaric bupivacaine + clonidine 75 μg + saline (n=26) Group 2: hyperbaric bupivacaine + fentanyl 12.5 μg + clonidine 75 μg (n=26) | At spinal anaesthesia | SBP<100 mm Hg | |
| Pan and colleagues30 | Taiwan | Spinal | Hyperbaric bupivacaine 10 mg | Intrathecal | 150 μg | Hyperbaric bupivacaine (n=20) | Hyperbaric bupivacaine + clonidine 150 μg (n=20) | At spinal anaesthesia | Meperidine – regime not described | SBP below 100 mmHg |
| Braga and colleagues22 | Brazil | CSE | Hyperbaric bupivacaine 10 mg | Intrathecal | 75 μg | Hyperbaric bupivacaine (n=24) | Hyperbaric bupivacaine + clonidine 75 μg (n=24) | At spinal anaesthesia | Tenoxicam 40 mg, dipyrone 30 mg kg−1 VAS>3 in PACU | SBP<20% of baseline or SBP< 100 mm Hg |
| Singh and colleagues32 | India | Spinal | Hyperbaric bupivacaine 10 mg | Intrathecal | 50, 75 μg | Hyperbaric bupivacaine + fentanyl 25 μg (n=35) | Group 1: hyperbaric bupivacaine + clonidine 50 μg (n=35) Group2: hyperbaric bupivacaine + clonidine 75 μg (n=35) | At spinal anaesthesia | Intramuscular diclofenac 1.5 mg kg−1 | 20% decrease from baseline SBP |
| Khezri and colleagues36 | Iran | Spinal | Bupivacaine 10 mg | Intrathecal | 75 μg | Bupivacaine + 0.5 ml sterile water (n=30) | Bupivacaine + 75 μg clonidine (n=30) | At spinal anaesthesia | Diclofenac sodium 100 mg rectally every 8 h Pethidine 25 mg i.v. prn | SBP<20% below baseline, SBP<90 mm Hg |
| Cho and colleagues 200324 | Korea | Spinal | Hyperbaric bupivacaine 8 mg | Intrathecal | 75 μg | Hyperbaric bupivacaine + 0.55 ml 0.9% saline (n=20) | Hyperbaric bupivacaine + clonidine 75 μg (n=20) | At spinal anaesthesia | Not described | SBP<100 mm Hg |
| Shidhaye and colleagues31 | India | Spinal | Hyperbaric bupivacaine 10 mg | Intrathecal | 60 μg | Hyperbaric bupivacaine + fentanyl 25 μg (n=20) | Hyperbaric bupivacaine + clonidine 60 μg (n=20) | At spinal anaesthesia | Intramuscular diclofenac 75 mg (administered when VAS>7) | >20% reduction in baseline blood pressure |
| Li and colleagues35 | China | CSE | Hyperbaric bupivacaine 10 mg | Intrathecal | 75 μg | Hyperbaric bupivacaine + 0.9% saline (n=21) | Hyperbaric bupivacaine + clonidine 75 μg (n=21) | At spinal anaesthesia | Not described | 20% fall in pre-induction SBP |
| Bhattacharjee and colleagues37 | India | Spinal | Hyperbaric Bupivacaine 10 mg | Intrathecal | 75 μg | Hyperbaric bupivacaine + 0.9% saline (n=30) | Hyperbaric bupivacaine + clonidine 75 μg (n=30) | At spinal anaesthesia | Not described | Not described |
| Capogna and colleagues23 | Italy | Epidural | Epidural 2% lidocaine with epinephrine 1:800 000 | Epidural | 75,150 μg | 10 ml solution containing 2 mg morphine diluted with 0.125% bupivacaine + 1:800 000 epinephrine (n=20) | Group 1: 10 ml solution containing 2 mg morphine diluted with 0.125% bupivacaine + 1:800 000 epinephrine + clonidine 75 μg (n=20) Group 2: 10 ml solution containing 2 mg morphine diluted with 0.125% bupivacaine + 1:80 000 epinephrine + clonidine150 μg (n=20) | End of surgery | 10 ml solution containing 2 mg morphine diluted with 0.125% bupivacaine + 1:800 000 epinephrine + 0, 75, 150 μg clonidine was repeated on patient's request up to 36 h postoperatively | |
| Onat and colleagues28 | Turkey | Epidural | Epidural bupivacaine 0.5% (16 mL) | Epidural | 150 μg | Epidural bupivacaine 0.5% + fentanyl 50 μg (n=20) | Epidural bupivacaine 0.5% + clonidine 150 μg (n=20) | At placement of epidural catheter | Not described | SBP<30% of baseline |
| Bajwa and colleagues34 | India | Epidural | Epidural 0.75% ropivacaine (20 ml) | Epidural | 75 μg | Epidural 0.75% ropivacaine (n=24) | Epidural 0.75% ropivacaine + clonidine 75 μg (n=27) | At placement of epidural catheter | At the onset of postoperative pain epidural 8 mL of 0.175% ropivacaine was administered in the control group vs 0.175% + clonidine 50 μg in the intervention group | 20% reduction in SBP |
| Massone and colleagues26 | Italy | CSE | Spinal isobaric bupivacaine 0.5% 2.7–3 mL + 250 μg preservative free morphine | Epidural | 150 μg | Epidural saline (n=20) | Epidural clonidine 150 μg (n=20) | At the end of surgery after sensory block regression | IV ketorolac 30 mg as requested | |
| Mendez and colleagues27 | USA | Epidural | Bupivacaine 0.5% | Epidural bolus followed by infusion | 400 μg bolus + 10 μg/h, 800 μg bolus + 20 μg/h | Epidural saline 10ml bolus + 2 mL/h for 24 h | Group 1: Epidural clonidine 400 μg bolus + 10 μg/h for 24 h (n=20) Group 2: Epidural clonidine 800 μg bolus + 20 μg/h for 24 h (n=20) | On admission to recovery room | PCA morphine | |
| Huntoon and colleagues25 | USA | Epidural | Either 3% 2-chloroprocaine or 0.5% bupivacaine | Epidural bolus followed by infusion | 400 μg bolus + 40 μg h−1, 800 μg bolus + 40 μg h−1 | Epidural saline 10 ml bolus + 2 ml h−1 for 24 h (n=10) | Group 1: epidural clonidine 400 μg bolus + 40 μg h−1 for 24 h (n=10) Group 2: epidural clonidine 800 μg bolus + 40 μg h−1 for 24 h (n=10) | On first request for analgesia in the recovery room | PCA morphine initiated 15 min after epidural bolus injection |
Table 2.
Summary of risk of bias assessments for the included studies.
| Study ID | Selection bias |
Performance bias |
Detection bias |
Attrition bias |
Reporting bias |
summary of risk of bias | |
|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding of participants and researchers | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | ||
| Lavand'homme and colleagues10 | Low | Low | Low | Low | Low | Low | Low |
| van Tuijl and colleagues33 | Low | Unclear | Unclear | Low | Unclear | Unclear | Unclear |
| Paech and colleagues29 | Low | Low | Low | Low | Low | Low | Low |
| Benhamou and colleagues21 | Low | Unclear | Low | Low | High | Unclear | High |
| Pan and colleagues 99830 | Low | Unclear | Low | Low | Unclear | Unclear | Unclear |
| Braga and colleagues22 | Unclear | Unclear | Low | Low | Low | Unclear | Unclear |
| Singh and colleagues32 | Low | Low | Low | Low | Low | Unclear | Unclear |
| Khezri and colleagues36 | Low | Low | Low | Low | Low | Low | Low |
| Cho and colleagues24 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Shidhaye and colleagues31 | Low | Low | Low | Low | Unclear | Unclear | Unclear |
| Li and colleagues35 | Unclear | Low | Low | Low | Unclear | Unclear | Unclear |
| Capogna and colleagues23 | Unclear | Unclear | Low | Low | Unclear | Unclear | Unclear |
| Bhattacharjee and colleagues37 | Low | Unclear | Low | Unclear | Low | Unclear | Unclear |
| Onat and colleagues28 | Unclear | High | High | High | Unclear | Unclear | High |
| Bajwa and colleagues34 | Low | Unclear | Low | Low | Low | Unclear | Unclear |
| Massone and colleagues26 | Unclear | High | High | High | Low | Low | High |
| Mendez and colleagues27 | Unclear | High | Low | Low | Unclear | Unclear | High |
| Huntoon and colleagues25 | Unclear | Unclear | Low | Low | Unclear | Unclear | Unclear |
24 h morphine consumption
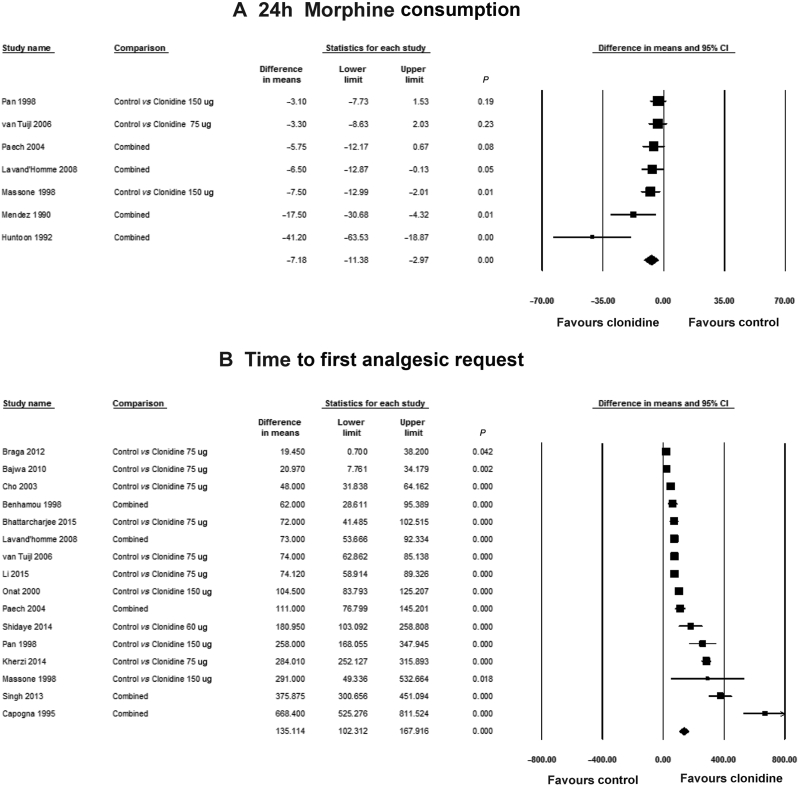
Seven studies10, 25, 26, 27, 29, 30, 33 reported 24 h analgesic consumption, with 194 patients in the control group vs 371 patients in the clonidine-treated groups. The postoperative analgesic regime is described in Table 1. Meperidine was administered as the postoperative analgesic in one study30 and i.v. ketorolac was used in another study.26 I.V. patient-controlled analgesia (PCA) with morphine was administered in the remaining five studies.10, 25, 27, 29, 33 Overall, the administration of clonidine reduced 24 h morphine consumption by 7.2 mg (95% CI: −11.4, −3.0 mg, I2: 61%) when compared with the placebo group (Fig. 2a). With a mean 24 h morphine consumption of 32.6 mg in the control group, this MD represents a 21% reduction in 24 h morphine consumption. When the studies with a high risk of bias were excluded,26, 27 the reduction in 24 h morphine consumption was still statistically significant [−6.19 mg (−11.12, −1.12 mg), I2: 65%].
Fig 2.
Pooled estimates of (a) 24 h morphine consumption and (b) time to first analgesic request in patients receiving neuraxial clonidine vs control. The combined comparisons represent studies in which multiple treatment arms were combined. CI, confidence interval.
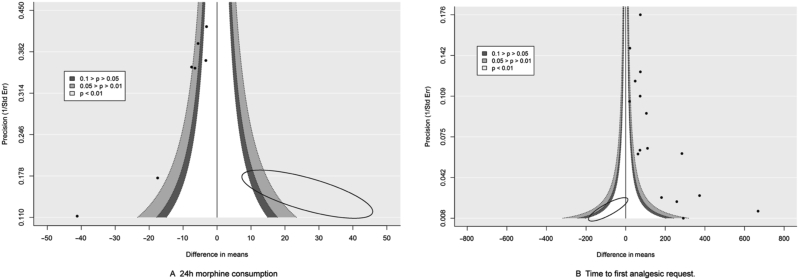
There was evidence of funnel plot asymmetry for 24 h morphine consumption [Intercept (95% CI) −3.816 ( −5.209, −2.426), P=0.001]. Examination of the contour enhanced funnel plot indicated that the missing studies were in the area of statistical significance 0.05>P>0.01 and P<0.01 (Fig. 3a). This suggests that the funnel plot asymmetry may be as a result of other factors apart from publication bias, such as the variable study quality of the studies included in the analysis.
Fig 3.
Contour enhanced funnel plot of (a) 24 h morphine consumption and (b) time to first analgesic request. The ellipse highlights areas where missing studies are expected.
The effect of intrathecal and epidural clonidine
When the analysis was restricted to studies where clonidine was administered only via the intrathecal route,10, 29, 30, 33 clonidine reduced 24 h morphine consumption by 4.3 mg (95% CI: −7.0, −1.5 mg, I2: 0%). Only in one study29 was clonidine co-administered via the intrathecal route with morphine and when this study was excluded from this subgroup analysis, the reduction in morphine consumption was still statistically significant [−3.9 mg (95% CI: −7.0, −0.9 mg, I2: 0%)]. When administered by the epidural route, clonidine significantly reduced morphine consumption by 18.9 mg (95% CI: −34.8, −3.0 mg, I2: 79%) when compared with placebo.25, 26, 27
Exclusion of studies where clonidine was co-administered with morphine
Overall when the two studies26, 29 where neuraxial clonidine was co-administered with morphine were excluded, clonidine still reduced morphine consumption by 8.7 mg (95% CI: −15.3, −2.0 mg, I2: 73%) when compared with the control group.
The effect of clonidine dose
There was no difference in the 24 h morphine consumption when we compared subgroups investigating doses of clonidine ≤75μg [MD (95% CI) −4.8mg (−10.1, 0.5 mg)] with those investigating doses >75 μg [MD (95% CI) −8.0 mg (−12.3, −3.7 mg)] (P=0.36).
Time to first analgesic request
Sixteen studies10, 21, 22, 23, 24, 26, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 reported the time to first analgesic request with 432 patients in the placebo group vs 664 patients receiving clonidine. Overall, the administration of clonidine increased the time to first analgesic request by 135 min (95% CI: 102, 168 min, I2: 96%) (Fig. 2b). When the studies with a high risk of bias were excluded,21, 26, 28 the administration of clonidine increased the time to first analgesic request by 150 min (95%CI: 110, 190 min, I2: 97%). There was evidence of funnel plot asymmetry for the time to first analgesic request [Intercept (95% CI) 6.166 (1.998, 10.334), P=0.007]. Examination of the contour-enhanced funnel plot indicated that the missing studies were in the area of statistical non-significance (Fig. 3b). This suggests that the funnel plot asymmetry may in fact be because of publication bias.
The effect of intrathecal and epidural clonidine
When the analysis was restricted to studies where clonidine was administered via the intrathecal route,10, 21, 22, 24, 29, 30, 31, 32, 33, 35, 36, 37 clonidine still prolonged the time to first analgesia request by 124 min (95% CI: 89, 160 min, I2: 96%). Exclusion of the study by Paech and colleagues29 where clonidine was co-administered with morphine via the intrathecal route still resulted in a significant increase in time to first request for analgesia by 126 min (95% CI 88, 164 min, I2: 97%). When clonidine was administered by the epidural route,23, 26, 28, 34 the time to first request for analgesia was prolonged by 218 min (95% CI: 111, 325 min, I2: 97%) when compared with the control group.
Exclusion of studies where clonidine was co-administered with morphine
When the three studies23, 26, 29 where clonidine was administered with morphine were excluded, clonidine administration still increased the time to first analgesic request by 114 min (95% CI: 82, 147 min, I2: 97%).
The effect of clonidine dose
There was no significant difference between subgroups in time to first analgesic request if clonidine was administered at a dose ≤75 μg [MD (95% CI) 128 min (90, 166 min)] when compared with doses >75 μg [MD (95% CI) 195 min (131, 259 min)] (P=0.077).
Postoperative pain scores
Four studies10, 24, 29, 30 reported pain scores on movement at 0–6 h. There was no significant difference in pain scores on movement at 0–6 h between the clonidine-treated and placebo groups [MD (95% CI) 0.8 (−0.4, 2.0), I2: 74%]. Five studies10, 24, 29, 30, 33 reported pain scores on movement at 6–24 h. Clonidine was administered via the intrathecal route in all these studies. There was no significant difference in pain scores on movement at 24 h between the clonidine-treated and placebo groups [MD (95% CI) 0.2 (−0.2, 0.6), I2: 0%]. Pain scores at rest were heavily skewed making data transformation and further quantitative analysis inappropriate.
Need for intraoperative analgesic supplementation/intraoperative pain
Seven studies10, 21, 22, 29, 30, 32, 33 reported the need for intraoperative analgesic supplementation. In all these studies, clonidine was administered by the intrathecal route. Clonidine was administered with local anaesthetic only in four studies.22, 30, 32, 33 It was co-administered with local anaesthetic and preservative-free morphine in one study,29 sufentanil in one study,10 and fentanyl in another study.21 Intrathecal clonidine significantly reduced the need for intraoperative supplementation when compared with placebo with 17.1% (39/227) of patients in the control group vs 3.2% (15/475) patients in the clonidine-treated groups requiring supplementation [OR (95% CI)=0.224 (0.076, 0.663), I2: 21%] with an NNT (95% CI) of 8 (5,11).
Hypotension
Twelve studies10, 22, 24, 28, 29, 30, 31, 32, 33, 35, 36, 37 reported the incidence of intraoperative hypotension after the administration of clonidine for anaesthesia for Caesarean section. Clonidine was administered via the intrathecal route in 11 studies10, 22, 24, 29, 30, 31, 32, 33, 35, 36, 37 and via the epidural route in one study.28 The definition of intraoperative hypotension for each study is described in Table 1. The administration of clonidine was associated with a significant increase in the incidence of intraoperative hypotension with an incidence in the control group of 33% (114/342) compared with 49% (260/525) in the clonidine-treated groups [OR (95%)=2.849 (1.363, 5.957), I2: 76%] (Table 3).
Table 3.
Maternal adverse effects of neuraxial clonidine. CI, confidence intervals; I2, heterogeneity; NNH, number needed to harm; OR, odds ratio.
| Outcome | Number of studies | Control group n/N (%) | Clonidine group n/N (%) | OR | 95% CI |
I2 | NNH (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| Intraoperative hypotension | 12 | 114/342 (33%) | 260/525 (49%) | 2.849 | 1.363 | 5.957 | 76% | 7 (4, 10) |
| Intraoperative nausea | 9 | 51/242 (21%) | 79/422 (18%) | 1.010 | 0.389 | 2.624 | 72% | |
| Intraoperative vomiting | 9 | 26/242 (11%) | 52/422 (12%) | 0.935 | 0.499 | 1.751 | 3% | |
| Intraoperative sedation | 5 | 25/135 (18%) | 82/232 (35%) | 2.355 | 1.016 | 5.459 | 23% | 6 (4, 12) |
| Postoperative sedation | 4 | 34/70 (46%) | 84/120 (70%) | 1.983 | 0.354 | 11.100 | 76% | |
| Intraoperative bradycardia | 7 | 8/189 (4%) | 16/260 (6%) | 1.410 | 0.554 | 3.590 | 0% | |
| Postoperative nausea | 5 | 33/134 (25%) | 29/134 (22%) | 0.806 | 0.436 | 1.487 | 0% | |
| Postoperative vomiting | 6 | 21/154 (14%) | 22/174 (13%) | 1.040 | 0.510 | 2.123 | 0% | |
| Pruritus | 12 | 77/322 (24%) | 122/529 (24%) | 0.657 | 0.265 | 1.628 | 54% | |
Vasopressor dose requirements
Seven studies10, 21, 24, 29, 30, 33, 36 reported vasopressor dose requirements intraoperatively. In all these studies, clonidine was administered via the intrathecal route. Ephedrine was used in six studies for treating established hypotension.21, 24, 29, 30, 33, 36 Paech and colleagues29 administered a prophylactic infusion of ephedrine to maintain blood pressure within 20% of baseline. In one study, ephedrine was co-administered with phenylephrine and the phenylephrine dose was converted into the equivalent i.v. ephedrine dose and the total dose combined.10 Overall there were no differences in the i.v. ephedrine dose equivalents between those patients receiving intrathecal clonidine and those receiving placebo [MD (95% CI) −0.6 mg (−4.5, 3.2 mg), I2: 82%].
Sedation
Five studies10, 21, 28, 32, 34 reported the incidence of sedation intraoperatively. In three studies clonidine was administered via the intrathecal route,10, 21, 32 and in two studies it was administered via the epidural route.28, 34 Clonidine significantly increased the incidence of sedation intraoperatively when compared with placebo [(OR (95%)=2.355 (1.016, 5.459), I2: 23%] (Table 3). Four studies23, 25, 26, 27 reported the incidence of postoperative sedation. In all four studies, clonidine was administered via the epidural route at the end of surgery. The postoperative analgesic regimes are highlighted in Table 1. The effect of clonidine on postoperative sedation was inconclusive because of the wide 95% CI (Table 3).
Bradycardia
Seven studies10, 28, 31, 32, 34, 36, 37 reported the incidence of bradycardia intraoperatively. In two studies, clonidine was administered via the epidural route.28, 34 Bradycardia was defined as a heart rate <45 beats min−1 in one study,10 heart rate <50 beats min−1 in two studies,32, 36 heart rate <60 beats min−1 in one study,31 heart rate <55 beats min−1 in one study,34 and a heart rate <15 beats min−1 in another study.28 We attempted to contact the authors of this latter study for clarification regarding their published definition of bradycardia, but did not receive a response. Bradycardia was not defined in one study.37 The effect of clonidine on intraoperative bradycardia when compared with the placebo group was inconclusive because of the wide 95% CI (Table 3).
Nausea and vomiting
Nine studies21, 24, 28, 29, 31, 32, 34, 36, 37 reported the incidence of intraoperative nausea and intraoperative vomiting (Table 3). Five studies24, 26, 30, 33, 35 reported the incidence of postoperative nausea and six studies23, 24, 26, 30, 33, 35 reported the incidence of postoperative vomiting (Table 3). The effect of clonidine on intraoperative nausea, intraoperative vomiting, postoperative nausea, and postoperative vomiting were inconclusive because of the wide 95% CI.
Pruritus
Twelve studies21, 23, 25, 26, 28, 29, 31, 32, 33, 35, 36, 37 reported the incidence of pruritus during the perioperative period. Neuraxial morphine was administered in three studies.23, 26, 29 In the study by Paech and colleagues,29 the patients receiving rescue antipruritics were considered as having pruritus. The effect of clonidine on the incidence of perioperative pruritus was inconclusive because of the wide 95% CI (Table 3).
Respiratory depression
Eight studies22, 25, 27, 28, 30, 31, 35, 36 reported the incidence of postoperative respiratory depression. These results are reported qualitatively. Respiratory depression was clearly defined in only three studies. However, in none of these studies was clonidine co-administered with neuraxial morphine. Only in one study investigating the analgesic efficacy of clonidine where clonidine was administered as a postoperative epidural infusion were there any reported cases of respiratory depression.25 In this study, the authors reported a single case in the control group only. Overall, there were no cases of respiratory depression in the clonidine-treated groups (0/195) and only one patient had an episode of respiratory depression in the control group for an incidence of 0.01% (1/165).
Neonatal outcomes
Fetal umbilical artery cord pH was reported in four studies.21, 32, 33, 35 In these studies, clonidine was administered via the intrathecal route as part of the anaesthetic technique for Caesarean section. There was no difference in umbilical artery pH between the clonidine-treated and the placebo groups [MD (95% CI) 0.053 (−0.187, 0.293), I2: 0%].
Apgar scores at 1 and 5 min were reported in six studies.21, 29, 31, 32, 33, 35 In all six studies, clonidine was administered via the intrathecal route as part of the anaesthetic technique for Caesarean section. There were no differences in Apgar scores at 1 min [MD (95% CI) 0.113 (−0.016, 0.242), I2: 0%] or 5 min [MD (95% CI) −0.007 (−0.045, 0.032), I2: 0%] between the patients receiving intrathecal clonidine and those receiving placebo.
Discussion
The results of our meta-analysis highlight several key findings. The administration of neuraxial clonidine was associated with an improvement in postoperative analgesia as evidenced both by the modest reduction in i.v. morphine consumption at 24 h and the prolongation of time to first analgesic request. These outcomes were not influenced by the dose of clonidine administered. Despite this modest improvement in postoperative analgesia, there was no observed reduction in opioid related side effects. The administration of clonidine also reduced the need for intraoperative analgesic supplementation but increased the incidence of intraoperative hypotension and sedation. Finally, the administration of neuraxial clonidine did not adversely affect neonatal outcomes.
In the general surgical population, clonidine is known to exert analgesic effects when administered neuraxially.8, 9 In fact, two previous meta-analyses have investigated this effect, but one included studies where clonidine was co-administered with morphine and the majority of studies included patients also having general anaesthesia.8, 9 In both meta-analyses, patients undergoing Caesarean section made up the minority of articles included (two studies in one review, one study in the other). Despite this difference in the patient population, our results were comparable to both meta-analyses and provide evidence that clonidine enhances postoperative analgesia in women after Caesarean section. It is important to highlight that the reductions in opioid consumption and the prolongation of postoperative analgesia were modest and may not be clinically relevant. The limited clinical effect of neuraxial clonidine on postoperative analgesia is further highlighted by the fact that neuraxial clonidine administration did not significantly reduce pain scores on movement. This inability to demonstrate a reduction in the pain scores may also reflect differences in the postoperative analgesic regimes, as there was significant variation in how postoperative pain was managed in the included studies. We also observed that neuraxial clonidine reduced opioid consumption and prolonged postoperative analgesia even in the absence of long acting opioids such as intrathecal morphine. Neuraxial morphine is widely used in developed countries, but it is still unclear whether the addition of clonidine to neuraxial morphine will further enhance its analgesic efficacy after Caesarean section. The studies included tested a range of doses, but interestingly we were unable to demonstrate any significant differences between the clonidine doses ≤75 μg and doses >75 μg for both 24 h morphine consumption and time to first analgesic request. The ideal dose for clonidine that improves analgesia has not been established. However, in the absence of any clear evidence that higher doses are more efficacious at improving analgesia than lower doses, the minimal effective dose for analgesia would be appropriate.
Clonidine's predominant analgesic effect is mediated through spinal α2 adrenergic receptors and there is some evidence that this effect may be enhanced in pregnancy.6, 39, 40 However, clonidine may also mediate some of its effects by increasing acetylcholine concentrations in cerebrospinal fluid.41 Cholinergic activation of dorsal sensory neurons produces analgesic effects. Clonidine also provides analgesia for visceral pain and slows regression of the sensory block as reflected by the prolonged duration of analgesia in patients receiving a single dose of clonidine at the start of surgery.8 This ability of clonidine to enhance the sensory block may explain not only its postoperative analgesic effect, but also the reduced need for intraoperative analgesic supplementation when it was administered as part of the neuraxial anaesthetic technique for Caesarean section. Importantly in the majority of studies investigating the need for intraoperative analgesic supplementation during Caesarean section, intrathecal clonidine was co-administered with local anaesthetic only, which is not standard practice in developed countries. In fact, the more common practice of co-administering short and intermediate acting lipophilic opioids with local anaesthetic intrathecally enhances intraoperative analgesia and reduces the need for intraoperative analgesic supplementation.42, 43 It is not clear if the addition of clonidine to a local anaesthetic and opioid mixture would confer any additional improvement in intraoperative analgesia.
The administration of neuraxial clonidine significantly increased the incidence of intraoperative sedation. The sedative effect of α2 agonists may result from a supraspinal action inhibiting neuronal activity at the locus coeruleus in the medulla.44, 45 When administered neuraxially, this sedative effect may result from rostral spread of clonidine.46 In the postoperative period, the non-significant increase in the incidence of sedation observed in patients receiving neuraxial clonidine could reflect an enhancement of the sedative effect of morphine co-administered i.v. or via the neuraxial route. In two of the studies, high doses of epidural clonidine as a bolus followed by continuous infusion were co-administered with PCA morphine after operation.25, 27 In one study, epidural clonidine was administered postoperatively in patients who had also received high dose intrathecal preservative morphine as a part of their anaesthetic technique,26 and in another repeated boluses of clonidine and preservative free morphine were co-administered epidurally in the postoperative period for pain.23 The administration of neuraxial clonidine as repeated boluses or as a high dose continuous infusion in conjunction with PCA morphine are not currently used postoperative analgesic regimes. Despite this, any increase in maternal sedation may be undesirable in the context of enhanced recovery protocols in current obstetric anaesthesia practice, as it could delay skin to skin contact, early initiation and continuation of breastfeeding, and prolong the length of stay in the post anaesthesia care unit.
Despite this sedative effect of clonidine, none of the studies reported an increase in the incidence of respiratory depression after operation. However, none of the studies reporting respiratory depression administered neuraxial morphine in conjunction with neuraxial clonidine. While neuraxial morphine may be associated with early onset and delayed respiratory depression and an increase in hypercapnia events, recent evidence suggests that the risk of clinically significant respiratory depression is extremely low in women receiving neuraxial morphine for post-caesarean analgesia.47, 48 It is also currently unclear whether clonidine would enhance the respiratory depression observed following neuraxial morphine administration. However, the increased sedation observed with neuraxial clonidine administration suggests that it could also enhance opioid-induced maternal respiratory depression and compromise maternal safety in the postoperative period.
Clonidine administration was associated with significant intraoperative hypotension, however there was no difference in vasopressor requirements and neonatal outcomes, suggesting that these episodes of hypotension were not clinically relevant. The administration of intrathecal clonidine for labour analgesia has been associated with a reduction in umbilical artery pH and this was attributed to feto-placental hypoperfusion secondary to maternal hypotension.11 In all the included studies that reported the incidence of intraoperative hypotension, vasopressors were administered to treat hypotension, but only in one study was a prophylactic ephedrine infusion administered.29 While hypotension may be a concern with neuraxial clonidine administration, this adverse effect can be easily mitigated with the administration of prophylactic vasopressors to prevent maternal hypotension and reduce related side effects.49, 50, 51
Our systematic review has several limitations. Overall, several of the studies included were small clinical trials with unclear or high risk of bias limiting the validity of our findings. Limiting the analysis to studies with low risk of bias, however, did not alter our main findings. Despite clonidine being used as an analgesic adjunct, the majority of studies did not report on opioid consumption or pain scores, making it difficult to make robust recommendations on the analgesic effects of clonidine in the clinical setting. The studies also used different postoperative analgesic regimes, which may have contributed to the heterogeneity seen in our primary outcomes. We pooled studies administering epidural and intrathecal clonidine based on the fact that for the majority of the studies, the doses of clonidine used were comparable. However, there is little evidence to determine whether the pharmacokinetic and pharmacodynamic effects of clonidine administered by the epidural and intrathecal routes are comparable. Additionally, the epidural modes of delivery used were very heterogeneous, justifying a subgroup analysis. However, the analgesic effects of clonidine administered via the epidural route would be of clinical significance in obstetrics, as it is a frequently used anaesthetic technique for Caesarean section. Even though clonidine may enhance local anaesthetics with shorter acting opioids, its synergistic analgesic effects with neuraxial morphine and its effects on opioid-induced respiratory depression are less clear. Unfortunately, only in three included studies23, 26, 29 was morphine used in conjunction with clonidine, and based on the small number of patients in the included trials, we could not specifically determine whether clonidine enhanced the analgesic effect of morphine or increased the risk of respiratory depression when compared with shorter acting opioids.
Given the widespread use of neuraxial morphine as an analgesic adjunct in Caesarean section, studies investigating the analgesic efficacy and safety of neuraxial morphine co-administered with clonidine are needed. The sedative effects of neuraxial clonidine co-administered with morphine on maternal bonding and initiation of breastfeeding also need to be addressed. Despite the modest analgesic effects seen with clonidine on postoperative analgesia, one area of emerging interest is its role in preventing wound hyperalgesia and resulting persistent pain after Caesarean section. Only one study included in this meta-analysis demonstrated that intrathecal clonidine at a dose of 150 μg reduced peri-incisional wound hyperalgesia, a surrogate marker for chronic incisional pain, at 48 h.10 Further studies are needed to determine if clonidine may have a role in reducing persistent pain after Caesarean section, particularly in high risk women. Additionally, the fact that neuraxial clonidine produces its analgesic effects independent of opioid-dependent pain pathways suggests that it may be a useful adjunct in patients who are opioid-dependent or on opioid agonists for opioid addiction treatment. Studies investigating the role of neuraxial clonidine in the postoperative management regime of this challenging patient population would be a significant contribution to the field.
Conclusion
In summary, our findings demonstrate that neuraxial clonidine modestly enhances postoperative analgesia in women having Caesarean section with neuraxial anaesthesia. These beneficial effects have to be balanced against the increased incidence of intraoperative hypotension and sedation that may compromise maternal safety and no significant reduction in opioid-related side effects. Based on these findings, clonidine may be a useful analgesic adjunct in women having Caesarean section under neuraxial anaesthesia. Additionally, our findings demonstrate that α2 agonists administered neuraxially may be an alternative mode of providing post-caesarean analgesia.
Authors' contributions
Study concept/design: all authors.
Data collection: T.K.A., B.M.M., R.Y.K.
Data analysis/interpretation, writing paper: T.K.A., A.S.H.
Revising paper: all authors.
Acknowledgements
We would like to acknowledge the assistance of Beverly Murphy and Connie Schardt who assisted with the database search strategies, and Victor Hasselblad PhD and Mary Cooter MS who provided statistical advice. We would also like to acknowledge the assistance of Sebnem Sevgen, Dr. EuiHyeok Kim, and Dr. Jennifer Dominguez who translated articles into English.
Editorial decision: September 05, 2017
Handling editor: J.G. Hardman
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bja.2017.11.085.
Declarations of interest
No conflicts of interest to declare.
Funding
This work was supported by the National Center for Advancing Translational Sciences of the US National Institutes of Health under Award Number KL2TR001115 and the National Institute of General Medical Sciences under Award Number 5T32 GM008600-20 to T.K.A. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Martin J.A., Hamilton B.E., Ventura S.J. Births: final data for 2009. Natl Vital Stat Rep. 2011;60:1–70. [PubMed] [Google Scholar]
- 2.Carvalho B., Cohen S.E., Lipman S.S., Fuller A., Mathusamy A.D., Macario A. Patient preferences for anesthesia outcomes associated with cesarean delivery. Anesth Analg. 2005;101:1182–1187. doi: 10.1213/01.ane.0000167774.36833.99. [DOI] [PubMed] [Google Scholar]
- 3.Eisenach J.C., Pan P.H., Smiley R., Lavand'homme P., Landau R., Houle T.T. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87–94. doi: 10.1016/j.pain.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadsden J., Hart S., Santos A.C. Post-cesarean delivery analgesia. Anesth Analg. 2005;101:S62–S69. doi: 10.1213/01.ANE.0000177100.08599.C8. [DOI] [PubMed] [Google Scholar]
- 5.Wasiluk I.M., Castillo D., Panni J.K., Stewart S., Panni M.K. Postpartum analgesia with dexmedetomidine in opioid tolerance during pregnancy. J Clin Anesth. 2011;23:593–594. doi: 10.1016/j.jclinane.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Filos K.S., Goudas L.C., Patroni O., Polyzou V. Intrathecal clonidine as a sole analgesic for pain relief after cesarean section. Anesthesiology. 1992;77:267–274. doi: 10.1097/00000542-199208000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Blaudszun G., Lysakowski C., Elia N., Tramèr M.R. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–1322. doi: 10.1097/ALN.0b013e31825681cb. [DOI] [PubMed] [Google Scholar]
- 8.Elia N., Culebras X., Mazza C., Schiffer E., Tramèr M.R. Clonidine as an adjuvant to intrathecal local anesthetics for surgery: systematic review of randomized trials. Reg Anesth Pain Med. 2008;33:159–167. doi: 10.1016/j.rapm.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Engelman E., Marsala C. Efficacy of adding clonidine to intrathecal morphine in acute postoperative pain: meta-analysis. Br J Anaesth. 2012;110:21–27. doi: 10.1093/bja/aes344. [DOI] [PubMed] [Google Scholar]
- 10.Lavand'homme P.M., Roelants F., Waterloos H., Collet V., Kock M.F. An evaluation of the postoperative antihyperalgesic and analgesic effects of intrathecal clonidine administered during elective cesarean delivery. Anesth Analg. 2008;107:948–955. doi: 10.1213/ane.0b013e31817f1595. [DOI] [PubMed] [Google Scholar]
- 11.Missant C., Teunkens A., Vandermeersch E., Van de Velde M. Intrathecal clonidine prolongs labour analgesia but worsens fetal outcome: a pilot study. Can J Anesth. 2004;51:696–701. doi: 10.1007/BF03018428. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.P.T., Altman D.G., Gøtzsche P.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice A.S.C., Lloyd J., Miller C.G., Bullingham R.E., O'Sullivan G.M. A double-blind study of the speed of onset of analgesia following intramuscular administration of ketorolac tromethamine in comparison to intramuscular morphine and placebo. Anaesthesia. 1991;46:541–544. doi: 10.1111/j.1365-2044.1991.tb09651.x. [DOI] [PubMed] [Google Scholar]
- 15.Shaheen P.E., Walsh D., Lasheen W., Davis M.P., Lagman R.L. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage. 2009;38:409–417. doi: 10.1016/j.jpainsymman.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Saravanan S., Kocarev M., Wilson R.C., Watkins E., Columb M.O., Lyons G. Equivalent dose of ephedrine and phenylephrine in the prevention of post-spinal hypotension in Caesarean section. Br J Anaesth. 2006;96:95–99. doi: 10.1093/bja/aei265. [DOI] [PubMed] [Google Scholar]
- 17.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:1–10. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61:991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Duval S., Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Benhamou D., Thorin D., Brichant J.F., Dailland P., Milon D., Schneider M. Intrathecal clonidine and fentanyl with hyperbaric bupivacaine improves analgesia during cesarean section. Anesth Analg. 1998;87:609–613. doi: 10.1097/00000539-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Braga A.A., Frias J.A.F., Braga F.S., Poterio G.B., Hirata E.S., Torres N.A. Spinal anesthesia for cesarean section. Use of hyperbaric bupivacaine (10mg) combined with different adjuvants. Rev Bras Anestesiol. 2012;62:775–787. doi: 10.1016/S0034-7094(12)70178-2. [DOI] [PubMed] [Google Scholar]
- 23.Capogna G., Celleno D., Zangrillo A., Costantino P., Foresta S. Addition of clonidine to epidural morphine enhances postoperative analgesia after cesarean delivery. Reg Anesth Pain Med. 1995;20:57–61. [PubMed] [Google Scholar]
- 24.Cho H.Y., Jang S.Y., Han Y.J., Choe H., Kim D.C. Hemodynamic and analgesic effects of intrathecal clonidine and neostigmine on bupivacaine spinal anesthesia in patients undergoing cesarean section. Korean J Anesthesiol. 2003;44:65–72. [Google Scholar]
- 25.Huntoon M., Eisenach J.C., Boese P. Epidural clonidine after cesarean section. Appropriate dose and effect of prior local anesthetic. Anesthesiology. 1992;76:187–193. doi: 10.1097/00000542-199202000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Massone M.L., Lampugnani E., Calevo M.G., Gandolfo A., Montobbio G., Fossa S. The effects of a dose of epidural clonidine combined with intrathecal morphine for postoperative analgesia. Minerva Anestesiol. 1998;64:289–296. [PubMed] [Google Scholar]
- 27.Mendez R., Eisenach J.C., Kashtan K. Epidural clonidine analgesia after cesarean section. Anesthesiology. 1990;73:848–852. doi: 10.1097/00000542-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Onat U., Kuvaki B., Gunerli A. Comparision of epidural bupivacaine and fentanyl with bupivacaine and clonidine in cesarean section. Turk Anesteziyoloji ve Reanimasyon. 2000;28:260–264. [Google Scholar]
- 29.Paech M.J., Pavy T.J., Orlikowski C.E. Postcesarean analgesia with spinal morphine, clonidine, or their combination. Anesth Analg. 2004;98:1460–1466. doi: 10.1213/01.ane.0000111208.08867.3c. [DOI] [PubMed] [Google Scholar]
- 30.Pan P.M., Huang C.T., Wei T.T., Mok M.S. Enhancement of analgesic effect of intrathecal neostigmine and clonidine on bupivacaine spinal anesthesia. Reg Anesth Pain Med. 1998;23:49–56. doi: 10.1016/s1098-7339(98)90110-9. [DOI] [PubMed] [Google Scholar]
- 31.Shidhaye R.V., Shah B.B., Joshi S.S., Deogaonkar S.G., Bhuva A.P. Comparison of clonidine and fentanyl as an adjuvant to intrathecal bupivacaine for spinal anaesthesia and postoperative analgesia in patients undergoing caesarian section. Sri Lankan J Anaesthesiol. 2014;22:15–20. [Google Scholar]
- 32.Singh R., Gupta D., Jain A. The effect of addition of intrathecal clonidine to hyperbaric bupivacaine on postoperative pain after lower segment caesarean section: a randomized control trial. Saudi J Anaesth. 2013;7:283–290. doi: 10.4103/1658-354X.115360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Tuijl I., Klei W.A., Werff D.B., Kalkman C.J. The effect of addition of intrathecal clonidine to hyperbaric bupivacaine on postoperative pain and morphine requirements after Caesarean section: a randomized controlled trial. Br J Anaesth. 2006;97:365–370. doi: 10.1093/bja/ael182. [DOI] [PubMed] [Google Scholar]
- 34.Bajwa S.J., Bajwa S.K., Kaur J. Comparison of epidural ropivacaine and ropivacaine clonidine combination for elective cesarean sections. Saudi J Anaesth. 2010;4:47–54. doi: 10.4103/1658-354X.65119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z., Tian M., Zhang C.Y. A randomised controlled trial to evaluate the effectiveness of intrathecal bupivacaine combined with different adjuvants (fentanyl, clonidine and dexmedetomidine) in caesarean section. Drug Res (Stuttg) 2015;65:581–586. doi: 10.1055/s-0034-1395614. [DOI] [PubMed] [Google Scholar]
- 36.Khezri M.B., Rezaei M., Delkhosh Reihany M., Haji Seid Javadi E. Comparison of postoperative analgesic effect of intrathecal clonidine and fentanyl added to bupivacaine in patients undergoing cesarean section: a prospective randomized double-blind study. Pain Res Treat. 2014;2014 doi: 10.1155/2014/513628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharjee A., Singh N.R., Singh S.S., Debbrama B., Debbarma P., Singh T.H. A comparative study of intrathecal clonidine and fentanyl along with bupivacaine in spinal anesthesia for caesarean section. J Med Soc. 2015;29:145–149. [Google Scholar]
- 38.Toledo P., McCarthy R.J., Ebarvia M.J., Huser C.J., Wong C.A. The interaction between epidural 2-chloroprocaine and morphine: a randomized controlled trial of the effect of drug administration timing on the efficacy of morphine analgesia. Anesth Analg. 2009;109:168–173. doi: 10.1213/ane.0b013e3181a40cf6. [DOI] [PubMed] [Google Scholar]
- 39.Iwasaki H., Collins J.G., Saito Y., Uchida H., Kerman-Hinds A. Low-dose clonidine enhances pregnancy-induced analgesia to visceral but not somatic stimuli in rats. Anesth Analg. 1991;72:325–329. doi: 10.1213/00000539-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Wolff M., Heugel P., Hempelmann G., Scholz A., Mühling J., Olschewski A. Clonidine reduces the excitability of spinal dorsal horn neurones. Br J Anaesth. 2007;98:353–361. doi: 10.1093/bja/ael379. [DOI] [PubMed] [Google Scholar]
- 41.De Kock M., Eisenach J., Tong C., Schmitz A.L., Scholtes J.L. Analgesic doses of intrathecal but not intravenous clonidine increase acetylcholine in cerebrospinal fluid in humans. Anesth Analg. 1997;84:800–803. doi: 10.1097/00000539-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 42.Cowan C.M., Kendall J.B., Barclay P.M., Wilkes R.G. Comparison of intrathecal fentanyl and diamorphine in addition to bupivacaine for Caesarean section under spinal anaesthesia. Br J Anaesth. 2002;89:452–458. [PubMed] [Google Scholar]
- 43.Dahl J.B., Jeppesen I.S., Jørgensen H., Wetterslev J., Møiniche S. Intra-operative and postoperative analgesic efficacy and adverse effects of intrathecal opioids in patients undergoing cesarean section with spinal anesthesia: a qualitative and quantitative systematic review of randomized controlled trials. Anesthesiology. 1999;91:1919–1927. doi: 10.1097/00000542-199912000-00045. [DOI] [PubMed] [Google Scholar]
- 44.Marwaha J., Kehne J.H., Commissaris R.L., Lakoski J., Shaw W., Davis M. Spinal clonidine inhibits neural firing in locus coeruleus. Brain Res. 1983;276:379–383. doi: 10.1016/0006-8993(83)90752-7. [DOI] [PubMed] [Google Scholar]
- 45.Bernard J.-M., Kick O., Bonnet F. Comparison of intravenous and epidural clonidine for postoperative patient-controlled analgesia. Anesth Analg. 1995;81:706–712. doi: 10.1097/00000539-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Post C., Gordh T., Jr., Minor B.G., Archer T., Freedman J. Antinociceptive effects and spinal cord tissue concentrations after intrathecal injection of guanfacine or clonidine into rats. Anesth Analg. 1987;66:317–324. [PubMed] [Google Scholar]
- 47.Crowgey T.R., Dominguez J.E., Peterson-Layne C., Allen T.K., Muir H.A., Habib A.S. A retrospective assessment of the incidence of respiratory depression after neuraxial morphine administration for postcesarean delivery analgesia. Anesth Analg. 2013;117:1368–1370. doi: 10.1213/ANE.0b013e3182a9b042. [DOI] [PubMed] [Google Scholar]
- 48.Bauchat J.R., McCarthy R., Fitzgerald P., Kolb S., Wong C.A. Transcutaneous carbon dioxide measurements in women receiving intrathecal morphine for cesarean delivery: a prospective observational study. Anesth Analg. 2017;124:872–878. doi: 10.1213/ANE.0000000000001751. [DOI] [PubMed] [Google Scholar]
- 49.Allen T.K., George R.B., White W.D., Muir H.A., Habib A.S. A double-blind, placebo-controlled trial of four fixed rate infusion regimens of phenylephrine for hemodynamic support during spinal anesthesia for cesarean delivery. Anesth Analg. 2010;111:1221–1229. doi: 10.1213/ANE.0b013e3181e1db21. [DOI] [PubMed] [Google Scholar]
- 50.Kee W.D.N., Khaw K.S., Ng F.F. Prevention of hypotension during spinal anesthesia for cesarean delivery: an effective technique using combination phenylephrine infusion and crystalloid cohydration. Anesthesiology. 2005;103:744–750. doi: 10.1097/00000542-200510000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka M., Balki M., Parkes R.K., Carvalho J.C.A. ED95 of phenylephrine to prevent spinal-induced hypotension and/or nausea at elective cesarean delivery. Int J Obstet Anesth. 2009;18:125–130. doi: 10.1016/j.ijoa.2008.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.