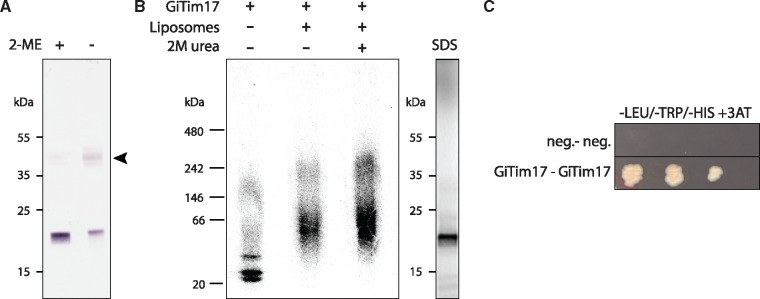
Fig. 3.
—GiTim17 forms dimers in the mitosomal membrane. (A) GiTim17 forms an ∼40 kDa complex on nonreducing SDS-PAGE. The complex depicted by the arrowhead brakes apart in the presence of reducing agent such as 2-mercapthoethanol (2-ME). (B) The complex of higher molecular weight corresponding approximately to the dimer of GiTim17 assembled in the liposomes upon in vitro translation. The complex was resistant to 2 M urea, which indicates its membrane insertion. Control SDS-PAGE of translated GiTim17 is shown on the right. (C) Mutual interaction of two GiTim17 proteins was positively tested in a yeast two hybrid assay under stringent conditions of 3-amino-1, 2, 4-triazole (3-AT).