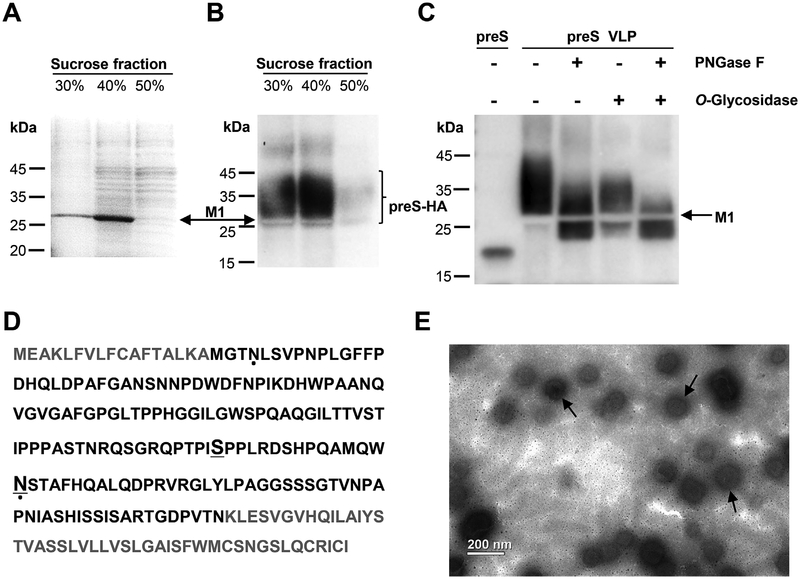
Fig. 2. Characterization of preS VLP.
(A) SDS-PAGE analysis of fractions from sucrose gradient centrifugation. The major protein component is in the 40% sucrose fraction. The position of M1 is indicated by the arrow. (B) Western blot analysis with purified polyclonal rabbit anti-preS antibody. Lanes 1 and 2 showed the presence of the preS-HA antigen in fractions from sucrose gradient centrifugation. The unstained region corresponds to M1. (C) The sample in 40% sucrose fraction was treated with Peptide -N-Glycosidase F (PNGase F) and/or O Glycosidase, and the preS antigen was detected by western blot. (D) Identification of glycosylated sites in preS-HA. The preS domain in preS-HA is colored black. Glycosylated residues identified in this study are underlined. Glycosylated residues which have been reported previously are indicated by dots (Lambert and Prange, 2007). (E) An electron micrograph showing preS virus-like particles (preS VLP). Magnification, 11,000 ×.