Fig. 2. Stereodivergent synthesis of SNAr precursors.
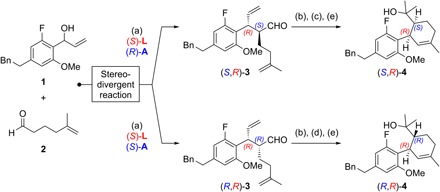
Reagents and conditions: (a) 1.0 equiv 1, 3.0 equiv 2, 3 mol% [{Ir(cod)Cl}2], 12 mol% (S)-L, 15 mol% (S)- or (R)-A, 5 mol% Zn(OTf)2, 1,2-dichloroethane (0.5 M), 25°C, 20 hours, for (S,R)-3: 59% yield, > 20:1 diastereomeric ratio (d.r.), >99% enantiomeric excess (e.e.), for (R,R)-3: 76% yield, > 12:1 d.r., >99% e.e.; (b) 5 mol% Grubbs II cat., CH2Cl2, 25°C, 16 hours; (c) 2.3 equiv NaClO2, 2.0 equiv NaH2PO4, 30 equiv 2-methyl-2-butene, tert-BuOH/H2O, 25°C; then 2.0 equiv Me3SiCHN2, C6H6/MeOH, 0°C, 90 min; (d) 8.0 equiv KOH, 3.9 equiv I2, MeOH, 0°C, 45 min; (e) 4.0 equiv MeMgBr, THF, 25°C, for (S,R)-4: 56% yield over three steps, for (R,R)-4: 56% yield over three steps; see the Supplementary Materials for structures of (S)-L, (S)-A, and (R)-A, as well as for further details.
