Fig. 3. Evolution of Cα3 and Nα3 helical content and conformational rearrangements within the Cα3 helix of CID domains during evolution.
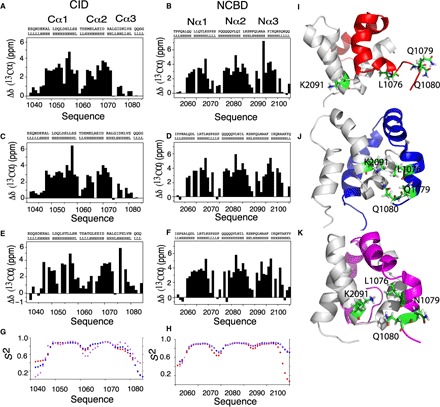
Plots of the difference of experimental Cα shifts from those of random coil values as a function of the amino acid sequences and TALOS prediction for ancestral and extant CID domains (A, C, and E) and NCBD domains (B, D, and F). The height of the bars indicates the degree of α helix formed with zero, indicating no α-helical content. There is a general increase in helical content for Cα3 and Nα3 as we evolve from Cambrian-like and Ordovician-Silurian (1R/2R) complexes to the present-day human CID/NCBD complex. (G and H) Chemical shift–based S2 values show that the flexibility in the Cα3 and Nα3 helices is higher in the most ancient low-affinity Cambrian-like complex (red) than in the younger high-affinity Ordovician-Silurian 1R/2R (blue) and present-day human (magenta) complexes. Specific interactions and rearrangements of K2091 of Nα2 and L1076, Q1079, and Q1080 of Cα3 (side chains are numbered and colored green) for (I) Cambrian-like D/P NCBD (gray) with 1R CID (red), (J) Ordovician-Silurian 1R/2R NCBD bound to 1R CID (blue), and (K) the extant human complex between CREBBP NCBD and NCOA3 CID (magenta). The three highlighted Cα3 residues are mainly solvent exposed in the most ancient Cambrian-like complex, while specific interactions are seen in both the Ordovician-Silurian 1R/2R and the extant human complex. For clarity, the structures have been slightly reoriented in relation to each other to show the specific interactions.
