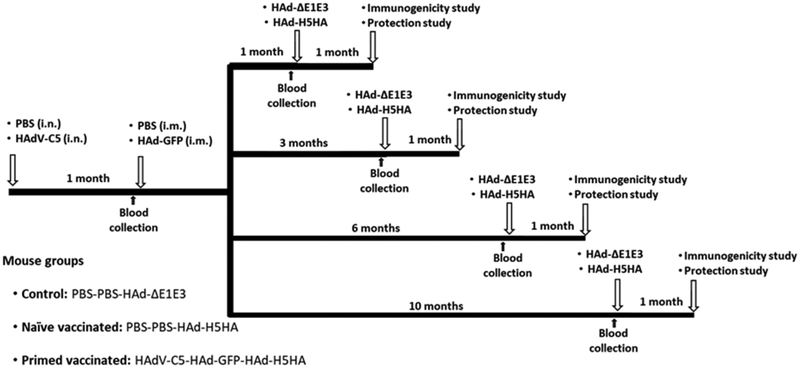
Fig. 1. Diagrammatic representation of animal immunization and challenge studies.
To mimic preexisting vector immunity and the development of vector-specific immune responses following first-time vaccination with an Ad vector-based vaccine, 5 to 6-week-old BALB/c mice were mock-inoculated (with PBS) or inoculated with 107 PFU of HAdV-C5 via the i.n. route (the natural route of HAdV-C5 infection in humans) to develop high levels (>200 virus-neutralizing antibody titers) of pre-existing vector immunity. At 1-month post-inoculation, the primed animals were inoculated i.m. with 108 PFU of HAd-GFP to mimic the conditions for a first inoculation with the AdV vector-based vaccine. Subsequently, at 1, 3, 6, and 10 months post-HAd-GFP inoculation, naïve- or HAdV-primed animals (10 mice/group) were vaccinated i.m. with 108 PFU of HAd-ΔE1E3 or HAd-H5HA. Before each immunization, blood samples were collected from the cheek vein. For immunogenicity studies, five animals from each group were euthanized under anesthesia at 4 weeks after immunization, and the blood and spleen were collected to monitor humoral and cell-mediated immune responses. For protection studies, the remaining five immunized animals were challenged i.n. with 100 mouse infectious dose 50 (MID50) of VN/1203/RG, euthanized at 3 days post-challenge, and the lungs were collected to determine the lung virus titers. PBS, phosphate buffer saline; i.n., intranasal; i.m., intramuscular; HAdV-C5, Human adenovirus type 5; HAd-ΔE1E3, HAdV-C5 empty vector with deletions in E1 and E3 regions; HAd-GFP, HAd-ΔE1E3 vector with the GFP gene inserted in the E1 region; HAd-H5HA, HAd-ΔE1E3 vector with the HA gene from A/HK/156/H5N1 influenza virus inserted in the E1 region.