Abstract
Objective:
Naltrexone has been shown to attenuate craving and the subjective effects of methamphetamine. Although naltrexone has modulatory effects on neural activity at dopaminergic synapses, the effect on striatal connectivity is unclear. As methamphetamine use is associated with greater resting-state functional connectivity (RSFC) in the dopaminergic system, we examined whether extended-release naltrexone (XR-NTX) can normalize striatal connectivity and whether changes in RSFC are associated with changes in craving and methamphetamine use.
Methods:
Thirty-seven participants in or seeking treatment for methamphetamine use disorder took part in this clinical trial at a university-based research clinic between May 2013 and March 2015 (Clinicaltrials.gov NCT01822132). Participants were randomized by a random number generator to a single four-week injection of XR-NTX or placebo. Functional magnetic resonance imaging (fMRI) and self-reported measures of craving and methamphetamine use were conducted before and after double-blinded randomization.
Findings:
There was a significant reduction in methamphetamine use in the naltrexone group and a significant treatment-by-time interaction on RSFC between the ventral striatum, amygdala, hippocampus, and midbrain. Connectivity was significantly reduced over time in participants randomized to naltrexone but unchanged in those randomized to placebo (p < 0.05, whole-brain corrected). Interactions between treatment and changes in connectivity show that significant reductions in connectivity were associated with reductions in methamphetamine use.
Conclusions:
Neurobiological deficits associated with methamphetamine use may undermine the efficacy of pharmacotherapies that directly target the dopamine reward system. Naltrexone, via antagonism of indirect mu-opioid effects on dopamine neurons, may attenuate reward system connectivity and aid in methamphetamine use treatment.
Keywords: Naltrexone, Resting-State fMRI, Methamphetamine, Striatum
1. Introduction
Behavioral approaches are the main treatments for methamphetamine (MA) use disorder, and although they can be effective, many patients relapse (Vocci and Appel, 2007). Despite a number of clinical trials testing the efficacy of agents including dopamine partial agonists and GABAergic and serotonergic agents, there are no approved pharmacological treatments for MA use disorder (Elkashef et al., 2008; Karila et al., 2010). Early abstinence from MA is accompanied by intense drug craving and high levels of cognitive impairment (Potvin et al., 2018; Zorick et al., 2010), which are important factors in the maintenance of addiction and may undermine treatment efficacy. Medications that interact with neural systems to attenuate craving and improve cognitive control may serve as a useful adjunct by enhancing the effectiveness of behavioral therapies.
The striatolimbic circuitry plays a central role in drug reinstatement and craving for stimulants (Garavan et al., 2000; McFarland et al., 2004; Volkow et al., 2008; Wong et al., 2006), underscoring the importance of the striatal dopamine system and its interactions with other transmitters in craving and susceptibility to substance use (London et al., 2015). There is considerable evidence of an interaction between the dopamine and opioid systems in which dopamine receptor agonists increase and antagonists decrease striatal mu-opioid receptors in animal models (Azaryan et al., 1996a). Similarly, administration of cocaine, a dopamine receptor agonist, increases mu-opioid receptor expression in the nucleus accumbens and amygdala (Azaryan et al., 1996b). Evidence of the effects of stimulants on the opioid system also come from human studies using positron emission tomography with [11C]Carfentanil to index endogenous opioid release through mu-opioid receptor binding. Administration of damphetamine increases endogenous opioid release in dorsal and ventral striatum and prefrontal cortex (Colasanti et al., 2012; Mick et al., 2014), and in people who use cocaine, greater muopioid receptor binding is positively correlated with craving for cocaine (Gorelick et al., 2005).
Naltrexone has shown promise for treating alcohol dependence, perhaps through opioid modulation of dopaminergic systems. Naltrexone exerts pharmacological effects primarily as a competitive mu-opioid receptor antagonist and modulating tonic GABAergic inhibition of midbrain dopaminergic neurons, thereby decreasing downstream dopamine release within the nucleus accumbens (Tambour and Quertemont, 2007). Greater dopamine release and metabolic activity in the ventral striatum is associated with an increase in amphetamine self-administration in rodents (Piazza et al., 1991). Naltrexone may, therefore, attenuate craving and subjective effects of MA in humans (Ray et al., 2015; Roche et al., 2017) and sensitization in animals (Chiu et al., 2005) by modulating neural activity at ventral striatal dopaminergic synapses. Although naltrexone has been shown to strengthen connectivity between the VTA and dorsal striatum but not the ventral striatum during a cue-processing task, this study aimed to investigate how extended release naltrexone (XR-NTX) affects the intrinsic activity of the ventral striatum at rest and the interactions with clinical outcomes such as craving and MA use in individuals with MA-use disorder. As MA use is associated with greater mesolimbic and striatolimbic resting-state functional connectivity (RSFC) (Kohno et al., 2018; Kohno et al., 2014; Kohno et al., 2016), we hypothesized that XR-NTX would reduce RSFC, MA craving, and MA use.
2. Materials and methods
This randomized clinical trial took place between May 2013 and March 2015. Participants were recruited from community-based treatment programs and primary care clinics in Portland, Oregon, U.S.A. Participants were eligible if they met DSM-IV criteria for Methamphetamine Dependence, had no other substance dependence except tobacco and/or nicotine dependence, no history of psychiatric disorder except depression and/or post-traumatic stress disorder, aspartate transaminase (AST) and alanine transaminase (ALT) < 5 times the upper limit of normal, were age 18 to 55 years, right handed, English-speaking, and free of drugs and alcohol > 72 hours prior to study assessments. Exclusion criteria included: opioid use in the last 30 days or opioid dependence in the past 5 years, a sensitivity to naltrexone, PLG (polylactide-co-glycolide) carboxymethylcellulose, or any other diluent components, a potential need for opioid analgesics during study period, pregnancy, magnetic resonance imaging (MRI) contraindications, or serious medical illness in the past 30 days.
2.1. Study design
All subjects provided written informed consent as approved by the Oregon Health and Science University and Veterans Affairs Portland Healthcare System Joint Institutional Review Board. Following consent, baseline resting-state functional MRI, survey assessments, and a negative urine toxicology screen for opioids (Visit 1) were acquired. A computerized random number generator was used to randomize participants to a single four-week injection of XR-NTX (Vivitrol, Alkermes) (n=19) versus identical-appearing placebo injection (n=18), both donated from the manufacturer. XR-NTX was chosen over daily-dosed oral naltrexone to ensure adherence to study medication and to allow reasonably sufficient time to observe potential changes in brain function following a single dose. Participants and study staff were blinded to randomization assignment. Survey and imaging assessments were repeated three weeks following injection (Visit 2).
2.2. Neuropsychiatric assessment
Participants were interviewed and rated by an experienced research assistant with the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998) to confirm substance dependence diagnoses and detect psychiatric disorders. Past 30-day substance use was assessed using the Addiction Severity Index-lite (ASI-lite) (Cacciola et al., 2007; McLellan et al., 1992). MA craving was assessed using a visual analogue scale (VAS) ranging from 0 (no craving) to 100 (most intense craving possible) (Hamilton et al., 2011).
2.3. MRI imaging acquisition
Imaging was performed on a 3 Tesla Siemens TIM Trio MRI scanner. A localizer scan was acquired in order to guide slice alignment during anatomical and functional scans. A T2*weighted image was acquired using an echo planar imaging scheme (EPI) (24 slices, 4 mm thick, gap width = 1 mm, TR/TE/α = 2,000 ms/38 ms/80°, matrix = 128×128, FOV = 240×240 mm, 170 volumes, in-plane pixel size of 1.875 mm2) while subjects stared at a white cross on a black screen for six minutes. One high-resolution T1-weighted magnetically prepared rapid acquisition gradient echo (MPRAGE; 144 slices 1 mm thick, TR/TE/TI/α = 2,300 ms/4.38 ms/1,200 ms/12°, FOV = 208×256 mm) was acquired for co-registration with functional images and statistical overlay.
2.4. Resting-state fMRI analysis
Image analysis was performed using FSL 5.0.2.1 (www.fmrib.ox.ac.uk/fsl). Images were realigned to compensate for motion with regression of three translational and three rotational parameters (Jenkinson et al., 2002). Data were skull-stripped, spatially smoothed (5 mm FWHM Gaussian kernel), and band-pass temporal filtered (.01–0.1 Hz). Further pre-processing included additional nuisance regressors: average signal of cerebrospinal fluid and white-matter and two metrics of motion-related artifact, specifically motion scrubbing with frame-wise displacement (FD) and a combination of the temporal derivative of the time series and root-mean-squared variance over all voxels (Power et al., 2011). To ensure no significant group differences in head movements, a t-test was used to compare groups on FD for each scan separately (Scan 1: p = 0.895; Scan 2: p = 0.984). Global signal regression was not applied. EPI images were registered to the MPRAGE and then into standard Montreal Neurological Institute space using a 12parameter affine transformation. An anatomically-defined region of interest from the Harvard-Oxford Subcortical atlas of the ventral striatum was used as a seed and transformed into each subject’s native space by applying the inverted transformation matrix of EPI to MPRAGE to standard space. The mean time series across all voxels within the ventral striatum seed from preprocessed images were used as a regressor in separate whole-brain, voxel-wise resting-state analyses. For a repeated measures group analysis, a two-way mixed effects analysis of variance (ANOVA) tested the main effect of time and the interaction of treatment and time on ventral striatal RSFC. To minimize false positives (Eklund et al., 2016), FSL’s local analysis of mixed effects (FLAME) was used where implicit estimation of variance is calculated using a Bayesian approach. All whole-brain RSFC statistics were corrected for multiple comparisons by using the Gaussian random fields theory with cluster-corrected statistics of voxel height threshold of Z > 2.3 and cluster significance of p < 0.05 (Worsley et al., 2011). This approach has been considered conservative, likely reducing false positives by incorporating first-level variances at the group level compared to standard random effects analysis including FSL’s ordinary least squares (OLS), standard SPM, and AFNI’s 3dttest++ where sphericity is assumed at the group level (Eklund et al., 2016).
2.5. Statistical analysis
Student’s t-tests and Fisher’s exact tests, where appropriate, were used to compare groups in baseline demographic and clinical variables (Table 1). Repeated measures ANOVA were used to examine the effects of XR-NTX on craving and MA use. The main effect of treatment (XRNTX or Placebo) and time (Visit 1 or Visit 2) and the interaction of treatment and time were examined on each measure separately. Following a significant group × time interaction with ventral striatal RSFC to the hippocampus and amygdala, a functionally-defined volume of interest of the hippocampus/amygdala cluster was used to extract average parameter estimates (β-values) from ventral striatal RSFC contrast maps, which correspond to the strength of functional connectivity with the ventral striatum as a function of treatment and time. An analysis of covariance was conducted to examine how changes in RSFC and treatment relate to changes in craving and MA use between visits using SPSS version 24 (IBM, Armonk, NY, USA). The analysis included and tested the main effect of group and the interaction between group and RSFC on changes in outcome measures of craving and MA use in two separate models.
Table 1.
Participant Characteristics
| Placebo (n = 19) | XR-NTX (n= 18) | p-value c | |
|---|---|---|---|
| Age (years) a | 36.47 ± 10.06 | 38.56 ± 9.55 | 0.523 |
| Sex (M/F) b | 14/5 | 14/4 | 0.772 |
| Education (yrs.) | 12.63 ± 0.83 | 12.78 ± 2.13 | 0.783 |
| Craving (VAS) | 23.84 ± 27.27 | 32.83 ± 27.69 | 0.327 |
| MA use | |||
| Days in the last 30 | 3.56 ± 6.42 | 5.06 ± 6.93 | 0.505 |
| Smoking | |||
| Number of smokers b | 17 | 14 | 0.335 |
| Cigarettes per day | 12.66 ± 7.16 | 8.14 ± 7.30 | 0.066 |
| Positive HIV Status b | 4 | 5 | 0.634 |
Data shown are means ± Standard Deviations
Data analyzed with Chi-squared test (X2)
No significant group differences in measures tested
3. Results
3.1. Participant characteristics
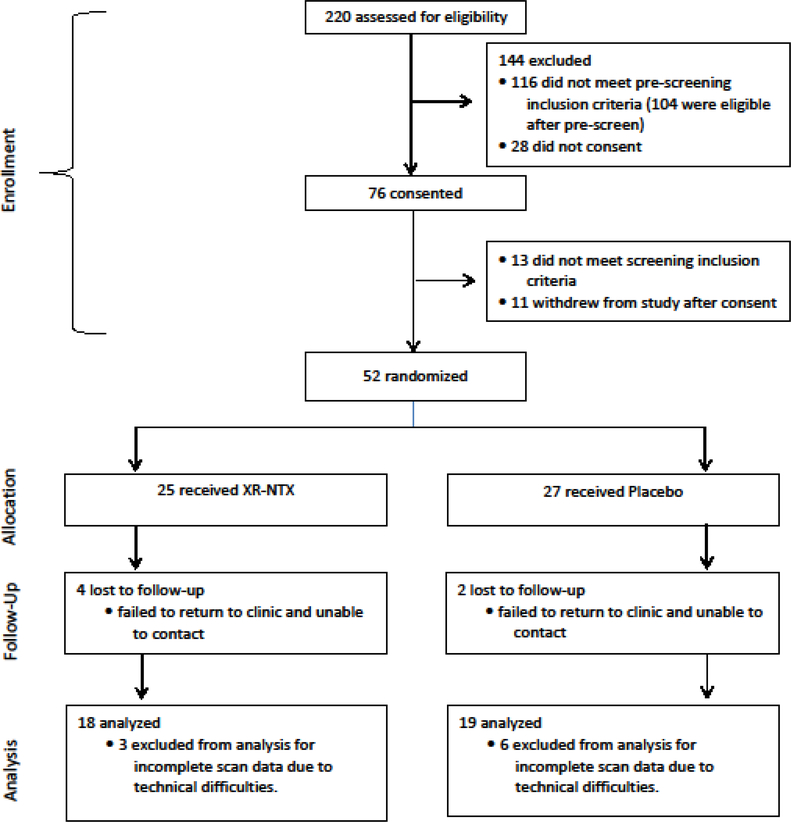
Research assistants pre-screened 220 individuals, 104 of whom were eligible for participation (Figure 1). The most common reasons for exclusion at pre-screening were polysubstance use, abstinence from MA for over six months, and MRI contraindications. Of the 104 eligible participants, 52 were randomized (50% of those who were eligible [23.6% of those screened]). Three eligible participants declined randomization. Of those randomized, 37 completed baseline and follow-up assessments that were available for analysis. Reasons for exclusion from analysis of those randomized included scheduling conflicts/no-shows and MRI confounds.
Figure 1. Study design.
Research assistants pre-screened 220 individuals, 104 of whom were eligible for participation. The most common reasons for exclusion at pre-screening were polysubstance use, clean from methamphetamine for too long, and MRI contraindications. Of the 104 eligible participants, 52 were randomized. Three eligible participants declined randomization. Of those randomized, 37 completed baseline and follow-up assessments that were available for analysis. Reasons for exclusion from analysis of those randomized included scheduling conflicts/no-shows and MRI confounds (artifacts, motion, did not complete task, etc.).
At baseline, groups were well-matched on demographic variables (Table 1). There were no significant differences in mean age (Placebo: 36.47 years; XR-NTX: 38.56 years, p = 0.523) or sex (Placebo: 73.7% men; XR-NTX: 77.8% men, p = 0.772). Both groups were similar in education and smoking status; a minimum of a high school or equivalent education was obtained in 94.4% and 100% of participants in the XR-NTX and Placebo group, respectively (p = 0.783), and cigarette smokers accounted for 77.8% of participants in the XR-NTX group and 89.5% of the Placebo group (p = 0.335).
There were no significant group differences in MA use in the 30 days prior to study enrollment (Placebo: 3.56 days; XR-NTX: 5.06 days, p = 0.505) or craving for MA indexed by the VAS (Placebo: 23.84; XR-NTX: 32.83, p = 0.327). HIV-positive participants accounted for 27.8% and 21.1% of the XR-NTX and Placebo group, respectively (p = 0.634). Among HIVpositive patients, the mean CD4 count was 408.75 ± 369.11 in the XR-NTX group compared to 701.5 ± 426.44 in the Placebo group (p = 0.339). One HIV-positive subject in each group had no current or past history of taking stable antiretroviral therapy, but all other HIV-positive patients were taking stable antiretroviral therapy prior to and during the study. CD4 and antiretroviral therapy information was not available for one subject in the XR-NTX Group.
3.2. Methamphetamine use and craving
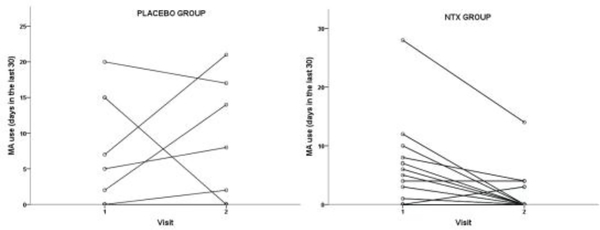
Mean number of days in the past 30 days of self-reported MA use decreased from 5.06 to 1.56 in the XR-NTX group and from 3.56 to 2.74 in the Placebo group. The repeated measures ANOVA resulted in a significant Time x Treatment interaction (p = 0.042), with the XR-NTX group showing greater significant reductions in MA use compared to the Placebo group (Figure 2). Mean craving scores decreased from 32.83 to 20.06 in the XR-NTX group and from 23.84 to 18.86 in the Placebo group. There was a significant main effect of time on craving scores (p = 0.043) and no significant Time x Treatment interaction (p = 0.384).
Figure 2. Group by time interaction on methamphetamine use.
A two-way repeated measures ANOVA was conducted to examine whether any change in MA use is the result of an interaction between two factors: Treatment Group (placebo group or naltrexone group) and Time (Visit 1 or Visit 2). MA use was the dependent variable and the main effect of Group (Placebo group, XTR-NTX group) and Time (visit 1, visit 2) and the interaction of Group and Time was tested. The Group x Time interaction shows that these two factors interact to produce changes in MA use, where the XTR-NTX group shows reductions in MA use from Visit 1 to Visit 2 (F(2,35)=4.80, p =0.04.
3.3. Resting-state functional connectivity
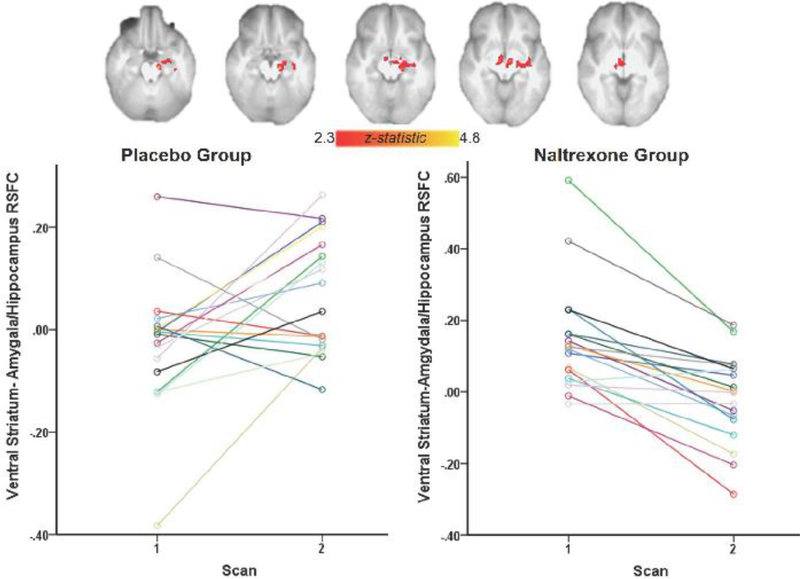
Whole-brain voxel-wise analysis revealed a main effect of time where there was an increase in connectivity across both groups between the ventral striatum and right middle and inferior frontal gyri, right ventral anterior insula, and right superior and inferior temporal gyri. There was also a significant interaction of time and treatment on RSFC, where the XR-NTX group but not the Placebo group exhibited a significant reduction in connectivity between ventral striatum, midbrain, left hippocampus, and amygdala between scans (p = 0.05, whole-brain corrected) (Figure 3).
Figure 3. Treatment by time interaction on ventral striatal RSFC.
The naltrexone group exhibited reduced connectivity between ventral striatum, amygdala, hippocampus and midbrain between scan 1 and scan 2 (p < 0.05, whole-brain corrected). Parameter estimates (regression β-values) were extracted from the functional ROI of the amygdala and hippocampus.
3.4. Relationship between RSFC and clinical outcome measures
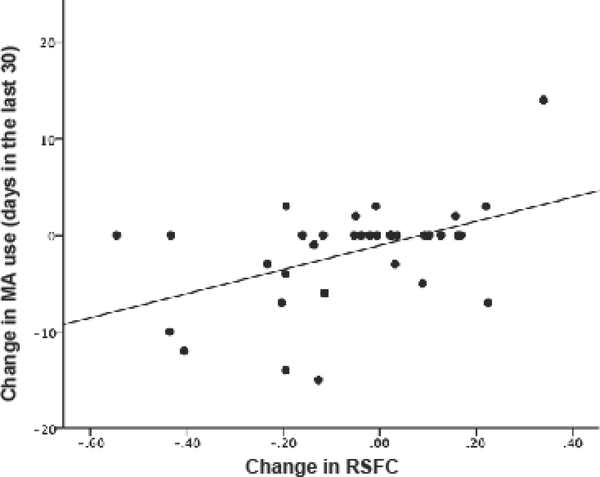
A significant main effect of RSFC (p = 0.003) and a group interaction (p = 0.039) between changes in RSFC of striatolimbic regions and changes in MA use was found: reduced RSFC was associated with a reduction in MA use. There were no significant main effects (p = 0.495) or group interactions (p = 0.388) between changes in RSFC and changes in craving.
4. Discussion
Neuroimaging studies of alcohol use disorder demonstrate the effects of naltrexone on brain function and connectivity (Lukas et al., 2013; Mann et al., 2014; Morris et al., 2017; Spagnolo et al., 2014). We extend these results to individuals with MA use disorder and show a reduction in striatolimbic RSFC in the XR-NTX group compared to a Placebo group. Although we find no significant group differences in the reduction of craving for MA, there is significant reduction in MA use in the XR-NTX group.
Endogenous opioid blockade has been used to treat impulsivity disorders such as eating disorders (Marrazzi et al., 1995; O’Malley et al., 2007), pathological gambling (Kim and Grant, 2001), kleptomania (Grant et al., 2009), and compulsive sexual behavior (Raymond et al., 2010) and facilitates relapse prevention for alcohol (Garbutt et al., 2005) and opioid (Krupitsky et al., 2011) dependencies. Early phase trials suggest that naltrexone, a potent mu opioid receptor antagonist, may decrease MA use. In a 12-week trial of naltrexone vs. placebo in 80 MA-dependent patients, those receiving once daily oral naltrexone achieve a greater mean percent of MA-negative urine drug screens (65.2% vs. 47.7%, p < 0.05) and report fewer days of MA use (5.5% vs. 16.1%, p < 0.05) (40). Urine testing of naltrexone metabolite 6-β-naltrexol demonstrated 62.5% adherence among patients assigned to naltrexone. Naltrexone efficacy would be expected to increase through use of XR-NTX, a once a month intramuscular injection. In a second study, 20 MA-dependent patients pre-treated with oral naltrexone experienced attenuated subjective effects and decreased craving when given dextroamphetamine, suggesting the subjective effects of MA may be partly mediated through the endogenous opioid system (Jayaram-Lindstrom et al., 2008a). A third, smaller study of 31 MA-dependent patients randomized to oral naltrexone vs. placebo demonstrated a non-significant trend toward reductions in MA craving or use during 8-weeks of follow-up but did not report information regarding adherence (Grant et al., 2010). Although our study shows a greater reduction in craving in the XR-NTX group, the effects do not meet statistical significance. This is similar to a recent study showing no effect of XR-NTX on MA craving among men who have sex with men (Coffin et al., 2018). The majority of participants from that trial were in residential treatment, which may explain why they found no reduction in MA use while our results show significant reductions with XR-NTX.
The XR-NTX group shows greater reduction in striatolimbic RSFC and in MA use than the Placebo group. These results are in line with studies of alcohol use disorder, where individuals who abstain from alcohol exhibit less RSFC in the reward/salience networks (Kohno et al., 2017). The reduction in striatolimbic RSFC extends findings of heightened RSFC between amygdala and hippocampus (Dean et al., 2014) and among regions of the mesocorticolimbic system (Kohno et al., 2014; Kohno et al., 2016) in MA use disorder and show that antagonizing mu-opioid receptors can reduce striatolimbic functional connectivity.
Dopamine signaling in the striatum is considered a key factor in the maintenance of stimulant use (Volkow et al., 2006; Wong et al., 2006). Evidence comes from reports that greater dopamine release and metabolic activity of the striatum and dopamine transmission in the nucleus accumbens is associated with an increase in amphetamine self-administration in rodents (Piazza et al., 1991). In addition, functional connectivity during a cue-reactivity paradigm is increased in the ventral tegmental area and dorsal striatum and decreased in the precuneus with naltrexone (Courtney et al., 2016). Neuroimaging studies also highlight the role of the ventral striatum in alcohol use disorder. Greater cue-induced activation of the striatum is associated with subsequent relapse in abstinent alcohol dependent subjects (Grusser et al., 2004). Furthermore, naltrexone decreases alcohol-cue induced activation of the ventral striatum (Myrick et al., 2008). Recently detoxified alcohol-dependent individuals exhibit greater activation of the ventral striatum when presented with alcohol-associated visual and olfactory cues (Braus et al., 2001; Grusser et al., 2004). Naltrexone reduces the subjective effects of amphetamines (Jayaram-Lindstrom et al., 2008b), perhaps through the downregulation of connectivity in striatolimbic dopaminergic regions, which may account for the greater reduction in drug use behavior in the XR-NTX group. This is consistent with the notion that the nucleus accumbens and amygdala play a substantial role in the reinstatement of drug-seeking behavior (Feltenstein and See, 2008) and that reactivity of amygdalar pathways may predict relapse and treatment efficacy (Koob and Volkow, 2010). Overall, these results collectively suggest that the reinstatement of drug-seeking behavior following exposure to discrete or contextual drug-associated cues involves DAergic and glutamatergic interactions between the NAcc core and the BLA and dmPFC.
The XR-NTX group shows greater reductions in craving compared to the Placebo group; however, the differences are not statistically significant. Furthermore, the XR-NTX-induced reductions in striatal RSFC with dopaminergic terminal regions are not associated with changes in craving. Although there is strong evidence for a relationship between dopamine signaling and drug craving, the association is often between cue-induced dopamine release with cue-induced craving: dopamine receptor occupancy or dopamine release is associated with cocaine cues and subsequent craving (Volkow et al., 2006; Wong et al., 2006). As changes in dopamine release alone do not induce craving unless paired with drug cues (Volkow et al., 2008), it is possible that the lack of significant findings with XR-NTX are due to measuring craving without drug cues in a laboratory setting. One study showed, however, that naltrexone affects functional connectivity during a cue-reactivity paradigm in a number of brain regions including dorsal striatum but not ventral striatum (Courtney et al., 2016). The lack of a relationship with ventral striatal resting-state connectivity is perhaps due to a stronger role of dorsal striatum in naltrexone-induced changes in craving. Future studies may consider examining the relationship between cue-induced craving and XR-NTX mediated changes in resting-state functional connectivity using different seed regions.
4.1. Limitations
Our findings should be interpreted in light of the following potential limitations. First, we enrolled a relatively small sample of participants with limited baseline MA use. Larger studies are needed to confirm and expand the current findings. In this study, we examined craving intensity using the VAS, a self-report measure of craving, but little is known about the temporal dynamics of these symptoms (Drummond, 2001). Also, the groups included HIV-positive individuals, and although groups were matched and almost all were on antiretroviral therapy prior to and during the study, HIV status could potentially confound the results. Lastly, this study was underpowered to examine sex by treatment interactions on RSFC and clinical variables. Sex differences, however, have been shown in incidence, prevalence, and outcomes for treatment (Hartung et al., 2002), and examining how sex may mediate differences in the effects of naltrexone on RSFC, craving, and MA use is important for future studies.
5. Conclusion
Naltrexone administration has been shown to reduce MA use in human clinical studies (Ray et al., 2015; Roche et al., 2017), and this study provides new evidence that naltrexone reduces striatolimbic RSFC in MA use disorder. We also highlight how naltrexone interacts with striatolimbic RSFC to reduce the use of MA, which is consistent with the hypothesis that engagement of the amygdalar pathway is necessary for subsequent drug reinstatement (Kalivas and McFarland, 2003; McFarland et al., 2004; Volkow et al., 2008). Future studies identifying the mechanism by which opioid antagonists alter the intrinsic connectivity of the striatolimbic system may aid in the development of adjunctive treatments for methamphetamine use disorder.
Figure 4. Relationship between change in ventral striatal RSFC and change in MA use.
Scatter plot shows the relationship between changes in RSFC between ventral striatum and amygdala/hippocampus and changes in days of MA use across all subjects. An increase in RSFC was associated with an increase in the number of days of MA use.
Highlights.
Naltrexone reduces methamphetamine (MA) use in those with MA use disorder.
Naltrexone reduces resting-state functional connectivity in striatolimbic networks.
Interactions show that reductions in connectivity relates to reductions in MA use.
Acknowledgements
The authors wish to thank Sarann Bielavitz and Doan Ha for assistance with the human subjects review. The study was funded by the U.S. National Institutes of Health, National Institute on Drug Abuse (R21DA033182, P50DA018165 07, UG1DA015815, T32DA007262 and T32AA007468) and Department of Veterans Affairs Clinical Sciences Research and Development Merit Review Program (WFH). The manufacturer, Alkermes, donated extendedrelease naltrexone and placebo injections for use in this trial. We thank all the study participants for their time and effort and the staffs of CODA Treatment Recovery and Volunteers of America Residential Treatment center.
Role of the Funding Source
Nothing declared
Footnotes
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azaryan AV, Clock BJ, Cox BM, 1996a. Mu opioid receptor mRNA in nucleus accumbens is elevated following dopamine receptor activation. Neurochem. Res 21, 1411–1415. [DOI] [PubMed] [Google Scholar]
- Azaryan AV, Coughlin LJ, Buzas B, Clock BJ, Cox BM, 1996b. Effect of chronic cocaine treatment on mu- and delta-opioid receptor mRNA levels in dopaminergically innervated brain regions. J. Neurochem 66, 443–448. [DOI] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grusser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A, 2001. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J. Neural Transm 108, 887–894. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, McLellan AT, Lin YT, Lynch KG, 2007. Initial evidence for the reliability and validity of a “Lite” version of the Addiction Severity Index. Drug Alcohol Depend. 87, 297–302. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Ma T, Ho IK, 2005. Attenuation of methamphetamine-induced behavioral sensitization in mice by systemic administration of naltrexone. Brain Res. Bull 67, 100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PO, Santos GM, Hern J, Vittinghoff E, Santos D, Matheson T, Colfax G, Batki SL, 2018. Extended-release naltrexone for methamphetamine dependence among men who have sex with men: A randomized placebo-controlled trial. Addiction 113, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti A, Searle GE, Long CJ, Hill SP, Reiley RR, Quelch D, Erritzoe D, Tziortzi AC, Reed LJ, Lingford-Hughes AR, Waldman AD, Schruers KR, Matthews PM, Gunn RN, Nutt DJ, Rabiner EA, 2012. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol. Psychiatry 72, 371–377. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA, 2016. The effects of pharmacological opioid blockade on neural measures of drug cue-reactivity in humans. Neuropsychopharmacology 41, 2872–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Kohno M, Hellemann G, London ED, 2014. Childhood maltreatment and amygdala connectivity in methamphetamine dependence: A pilot study. Brain Behav. 4, 867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC, 2001. Theories of drug craving, ancient and modern. Addiction 96, 33–46. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. USA 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J, 2008. Pharmacotherapy of methamphetamine addiction: An update. Subst. Abuse 29, 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE, 2008. The neurocircuitry of addiction: An overview. Br. J. Pharmacol 154, 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA, 2000. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry 157, 1789–1798. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW, 2005. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: A randomized controlled trial. JAMA 293, 1617–1625. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M, Endres CJ, Dannals RF, Frost JJ, 2005. Imaging brain mu-opioid receptors in abstinent cocaine users: Time course and relation to cocaine craving. Biol. Psychiatry 57, 1573–1582. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Odlaug BL, 2009. A double-blind, placebo-controlled study of the opiate antagonist, naltrexone, in the treatment of kleptomania. Biol. Psychiatry 65, 600606. [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Kim SW, 2010. A double-blind, placebo-controlled study of Nacetyl cysteine plus naltrexone for methamphetamine dependence. Eur. Neuropsychopharmacol 20, 823–828. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A, 2004. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology 175, 296–302. [DOI] [PubMed] [Google Scholar]
- Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, Hammond JA, Huggins W, Jackman D, Pan H, Nettles DS, Beaty TH, Farrer LA, Kraft P, Marazita ML, Ordovas JM, Pato CN, Spitz MR, Wagener D, Williams M, Junkins HA, Harlan WR, Ramos EM, Haines J, 2011. The PhenX toolkit: Get the most from your measures. Am. J. Epidemiol 174, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung CM, Willcutt EG, Lahey BB, Pelham WE, Loney J, Stein MA, Keenan K, 2002. Sex differences in young children who meet criteria for attention deficit hyperactivity disorder. J. Clin. Child Adolesc. Psychol 31, 453–464. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Hammarberg A, Beck O, Franck J, 2008a. Naltrexone for the treatment of amphetamine dependence: A randomized, placebo-controlled trial. Am. J. Psychiatry 165, 1442–1448. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J, 2008b. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology 33, 1856–1863. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K, 2003. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168, 44–56. [DOI] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL, 2010. Pharmacological approaches to methamphetamine dependence: A focused review. Br. J. Clin. Pharmacol 69, 578–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Grant JE, 2001. An open naltrexone treatment study in pathological gambling disorder. Int. Clin. Psychopharmacol 16, 285–289. [DOI] [PubMed] [Google Scholar]
- Kohno M, Dennis LE, McCready H, Hoffman WF, 2017. Executive control and striatal resting-state network interact with risk factors to influence treatment outcomes in alcohol-use disorder. Front. Psychiatry 8, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Loftis JM, Huckans M, Dennis LE, McCready H, Hoffman WF, 2018. The relationship between interleukin-6 and functional connectivity in methamphetamine users. Neurosci. Lett 677, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED, 2014. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry 71, 812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Okita K, Morales AM, Robertson CL, Dean AC, Ghahremani DG, Sabb FW, Rawson RA, Mandelkern MA, Bilder RM, London ED, 2016. Midbrain functional connectivity and ventral striatal dopamine D2-type receptors: Link to impulsivity in methamphetamine users. Mol. Psychiatry 21, 1554–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL, 2011. Injectable extended-release naltrexone for opioid dependence. Lancet 378, 665; author reply 666. [DOI] [PubMed] [Google Scholar]
- London ED, Kohno M, Morales AM, Ballard ME, 2015. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 1628, 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, Mallya G, Palmer C, Penetar DM, 2013. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: A bold FMRI study. Neuroimage 78, 176–185. [DOI] [PubMed] [Google Scholar]
- Mann K, Vollstadt-Klein S, Reinhard I, Lemenager T, Fauth-Buhler M, Hermann D, Hoffmann S, Zimmermann US, Kiefer F, Heinz A, Smolka MN, 2014. Predicting naltrexone response in alcohol-dependent patients: The contribution of functional magnetic resonance imaging. Alcohol. Clin. Exp. Res 38, 2754–2762. [DOI] [PubMed] [Google Scholar]
- Marrazzi MA, Markham KM, Kinzie J, Luby ED, 1995. Binge eating disorder: Response to naltrexone. Int. J. Obes 19, 143–145. [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW, 2004. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci 2, 1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M, 1992. The fifth edition of the Addiction Severity Index. J. Subst. Abuse Treat 9, 199–213. [DOI] [PubMed] [Google Scholar]
- Mick I, Myers J, Stokes PR, Erritzoe D, Colasanti A, Bowden-Jones H, Clark L, Gunn RN, Rabiner EA, Searle GE, Waldman AD, Parkin MC, Brailsford AD, Nutt DJ, Lingford-Hughes AR, 2014. Amphetamine induced endogenous opioid release in the human brain detected with [(1)(1)C]carfentanil PET: Replication in an independent cohort. Int. J. Neuropsychopharmacol 17, 2069–2074. [DOI] [PubMed] [Google Scholar]
- Morris LS, Baek K, Tait R, Elliott R, Ersche KD, Flechais R, McGonigle J, Murphy A, Nestor LJ, Orban C, Passetti F, Paterson LM, Rabiner I, Reed L, Smith D, Suckling J, Taylor EM, Bullmore ET, Lingford-Hughes AR, Deakin B, Nutt DJ, Sahakian BJ, Robbins TW, Voon V, Consortium I, 2017. Naltrexone ameliorates functional network abnormalities in alcohol-dependent individuals. Addict. Biol 23, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K, 2008. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch. Gen. Psychiatry 65, 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Sinha R, Grilo CM, Capone C, Farren CK, McKee SA, Rounsaville BJ, Wu R, 2007. Naltrexone and cognitive behavioral coping skills therapy for the treatment of alcohol drinking and eating disorder features in alcohol-dependent women: A randomized controlled trial. Alcohol. Clin. Exp. Res 31, 625–634. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H, 1991. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 567, 169–174. [DOI] [PubMed] [Google Scholar]
- Potvin S, Pelletier J, Grot S, Hebert C, Barr AM, Lecomte T, 2018. Cognitive deficits in individuals with methamphetamine use disorder: A meta-analysis. Addict. Behav 80, 154–160. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2011. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Courtney KE, Moallem NR, Lunny K, Roche D, Leventhal AM, Shoptaw S, Heinzerling K, London ED, Miotto K, 2015. The effects of naltrexone on subjective response to methamphetamine in a clinical sample: A double-blind, placebo-controlled laboratory study. Neuropsychopharmacology 40, 2347–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond NC, Grant JE, Coleman E, 2010. Augmentation with naltrexone to treat compulsive sexual behavior: A case series. Ann. Clin. Psychiatry 22, 56–62. [PubMed] [Google Scholar]
- Roche DJO, Worley MJ, Courtney KE, Bujarski S, London ED, Shoptaw S, Ray LA, 2017. Naltrexone moderates the relationship between cue-induced craving and subjective response to methamphetamine in individuals with methamphetamine use disorder. Psychopharmacology 234, 1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl. 20, 22–33; quiz 34–57. [PubMed] [Google Scholar]
- Spagnolo PA, Ramchandani VA, Schwandt ML, Zhang L, Blaine SK, Usala JM, Diamond KA, Phillips MJ, George DT, Momenan R, Heilig M, 2014. Effects of naltrexone on neural and subjective response to alcohol in treatment-seeking alcoholdependent patients. Alcohol. Clin. Exp. Res 38, 3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambour S, Quertemont E, 2007. Preclinical and clinical pharmacology of alcohol dependence. Fundam. Clin. Pharmacol 21, 9–28. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM, 2007. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction 102 Suppl. 1, 96–106. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C, 2006. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J. Neurosci 26, 6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C, 2008. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage 39, 1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED, 2006. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 31, 2716–2727. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Jezzard P, Mathews PM, Smith SM, 2011. Functional MRI: An introduction to methods: Statistical analysis of activation images. Oxford Scholarship Online, Oxford. [Google Scholar]
- Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G, Rawson R, London ED, 2010. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction 105, 1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]