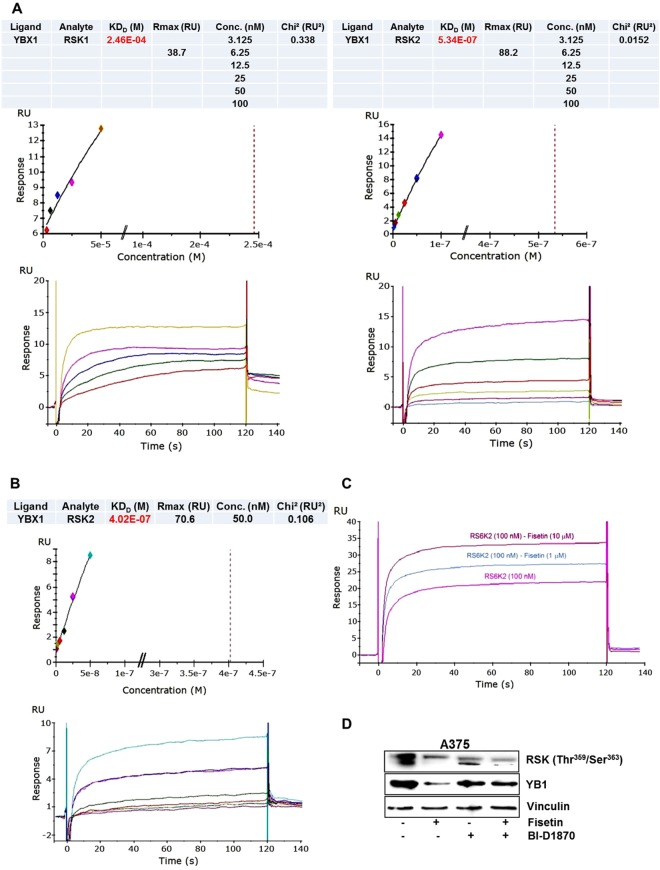
Figure 3.
Fisetin/RSK2 complex augments its binding to YB-1 (A) Representative SPR sensorgrams showing full kinetics for direct binding characteristics between YB-1 and RSK1 (left) or YB-1 and RSK2 (right). Briefly, YB-1 was directly immobilized on the sensor chip by amine coupling and RSK1 or RSK2 was flown over the protein-coated chip at different concentrations (100-0 nM). ka and kd or steady state kinetics were used for determining the KD. Chi square (χ2) analysis was carried out between the actual sensorgram (colored line, bottom) and the sensorgram generated from the BIAnalysis software (black line, top) to determine the accuracy of the analysis. χ2 value within 1–2 was considered significant (accurate), and below 1 was highly significant (highly accurate). (B) Representative sensorgram showing full kinetics between YB-1 and RSK2. In the presence of BSA, YB-1 was directly immobilized on the sensor chip by amine coupling and RSK2 was flown over the protein-coated chip at different concentrations (50-0 nM). (C) Representative sensorgram for competition assays, where fisetin and RSK2 were flown over immobilized YB-1. The concentration of RSK2 was kept constant (100 nM), but pre-incubated with increasing amounts of fisetin (1 and 10 µM) and then injected to determine the effect of fisetin on binding activity of the RSK2 to YB-1. Data for affinity evaluation were obtained from a concentration dependent binding curve for the interaction of increasing amounts of fisetin with constant concentration of RSK2. (D) Whole cell lysates of fisetin-treated A375 melanoma cells (60 μM:24 h) with/without RSK inhibitor BI-D1870 were analyzed for YB-1 expression. Equal loading was confirmed by reprobing for vinculin.