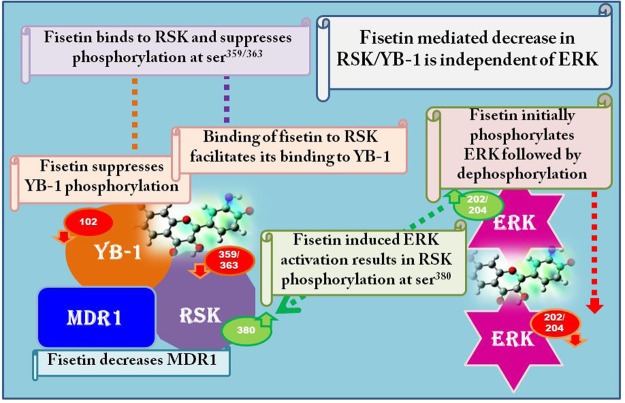
Figure 7.
Schematic diagram depicting the proposed mechanism of action of fisetin: SPR studies showed that interaction of fisetin with RSK2 promotes its binding to YB-1. Fisetin mediated downregulation of YB-1/RSK signaling is associated with a decrease in MDR1. Fisetin-treated cells showed an initial increase in ERK1/2 phosphorylation at threonine202/tyrosine204 followed by a sustained decrease, suggestive of a biphasic response. Fisetin mediated decrease in RSK/YB-1 is independent of ERK. Inhibition of ERK did not interfere with RSK phosphorylation at threonine359/serine363 residues in fisetin-treated cells. However, fisetin mediated increase in RSK serine380 phosphorylation was abrogated with ERK inhibition suggesting that ERK was driving phosphorylation at this residue.