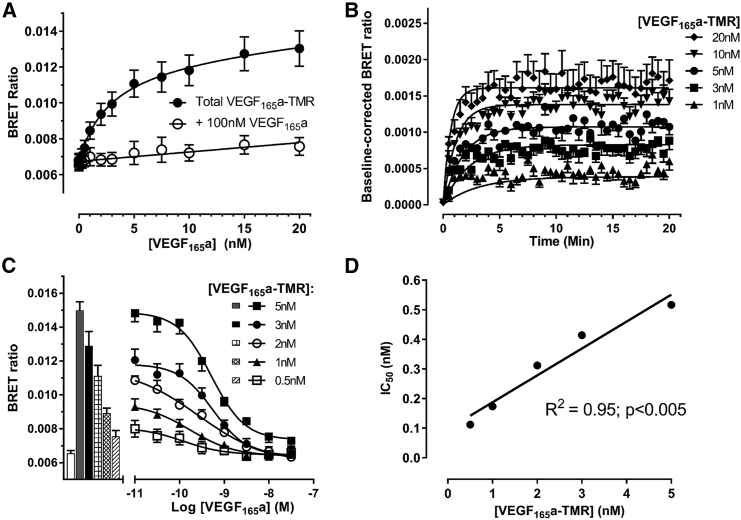
Figure 4.
Binding Characteristics of VEGF165a Binding to NanoLuc-NRP1
(A) Increasing concentrations of VEGF165a-TMR were added to HEK293T cells stably expressing N-terminal NanoLuc-NRP1 in the presence and absence of 100 nM unlabeled VEGF165a to determine non-specific binding, and cells were incubated for 60 min at 37°C. Raw BRET ratios are expressed as mean ± SEM from 5 independent experiments.
(B) Time course of VEGF165a-TMR binding to NanoLuc-NRP1. BRET ratios were baseline corrected to vehicle, curves were fitted to a simple exponential association model, and data are shown as mean ± SEM from 5 independent experiments.
(C) Inhibition of the binding of VEGF165a-TMR (0.5, 1, 2, 3, and 5 nM) to NanoLuc-NRP1 by increasing concentrations of unlabeled VEGF165a added simultaneously and incubated for 60 min at 37°C. Raw BRET ratios from 5 independent displacement experiments using duplicate wells are shown as mean ± SEM with bars representing vehicle (white) or VEGF165a-TMR only.
(D) Linear regression analysis (R2 = 0.95; p < 0.005) of the relationship between IC50 values determined in (C) and VEGF165a-TMR concentration. The y intercept provides an estimate for the Ki of competing VEGF165a (0.10 nM), while the slope (0.09) represents the ratio Ki/KD thus yielding an estimated KD = 1.11 nM for VEGF165a-TMR at NanoLuc-NRP1.