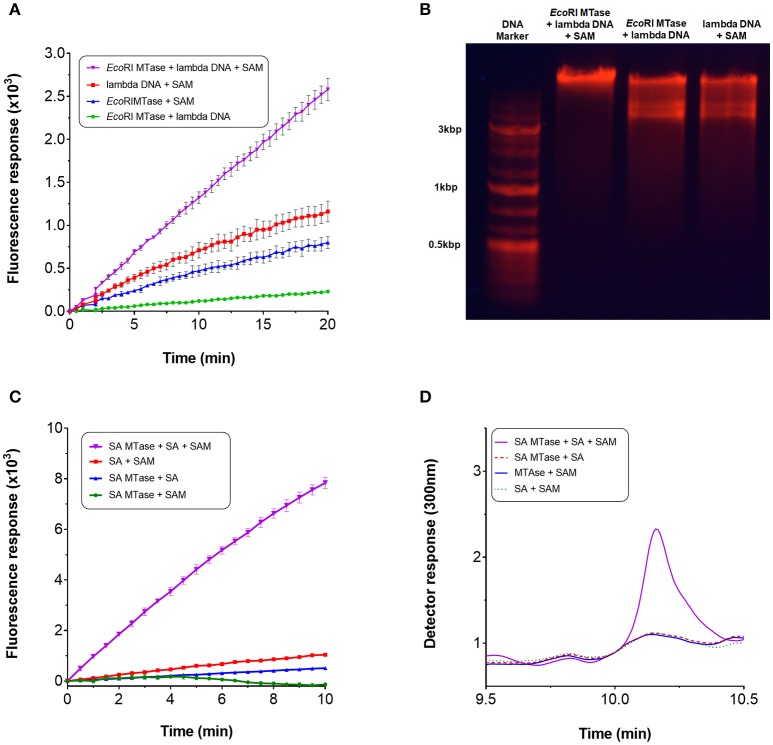
Figure 3.
Continuous monitoring of methyltransferases. To perform the methyltransferase assay, a 100 μl reaction typically contained the following assay components: 3.5 μg purified recombinant Mtn, 1 μg XOD (10 units/mg), 0.2 μg HRP (300 units/mg), 0.1 mM Amplex Red, 5 mM MgCl2, 0.5 mM SAM (32 mM stock), 1 mM salicylic acid or ~14 nM lambda DNA (0.5 mg/ml), 1-3 μg SA MTase or 80 units EcoRI MTase, and 50 mM Tris-HCl (pH 7.5) or 50 mM potassium phosphate buffer (pH 7.5). For SA MTase, the mixture was additionally supplemented with 1 mM KCl to stimulate activity. Reactions were initiated either with addition of the substrate or enzyme. Fluorescence output was monitored in 96-well microplates at 590 nm with excitation set at 530 nm. The reactions were incubated at 30°C without shaking for up to 60 mins. (A) Methyltransferase activity of EcoRI MTase in the presence of lambda DNA and SAM. (B) Confirmation of lambda DNA methylation by agarose gel electrophoresis. (C) Methyltransferase activity of SA MTase in the presence of salicylic acid (SA) and SAM. (D) Confirmation of the synthesis of methyl salicylate by HPLC.