Fig 4.
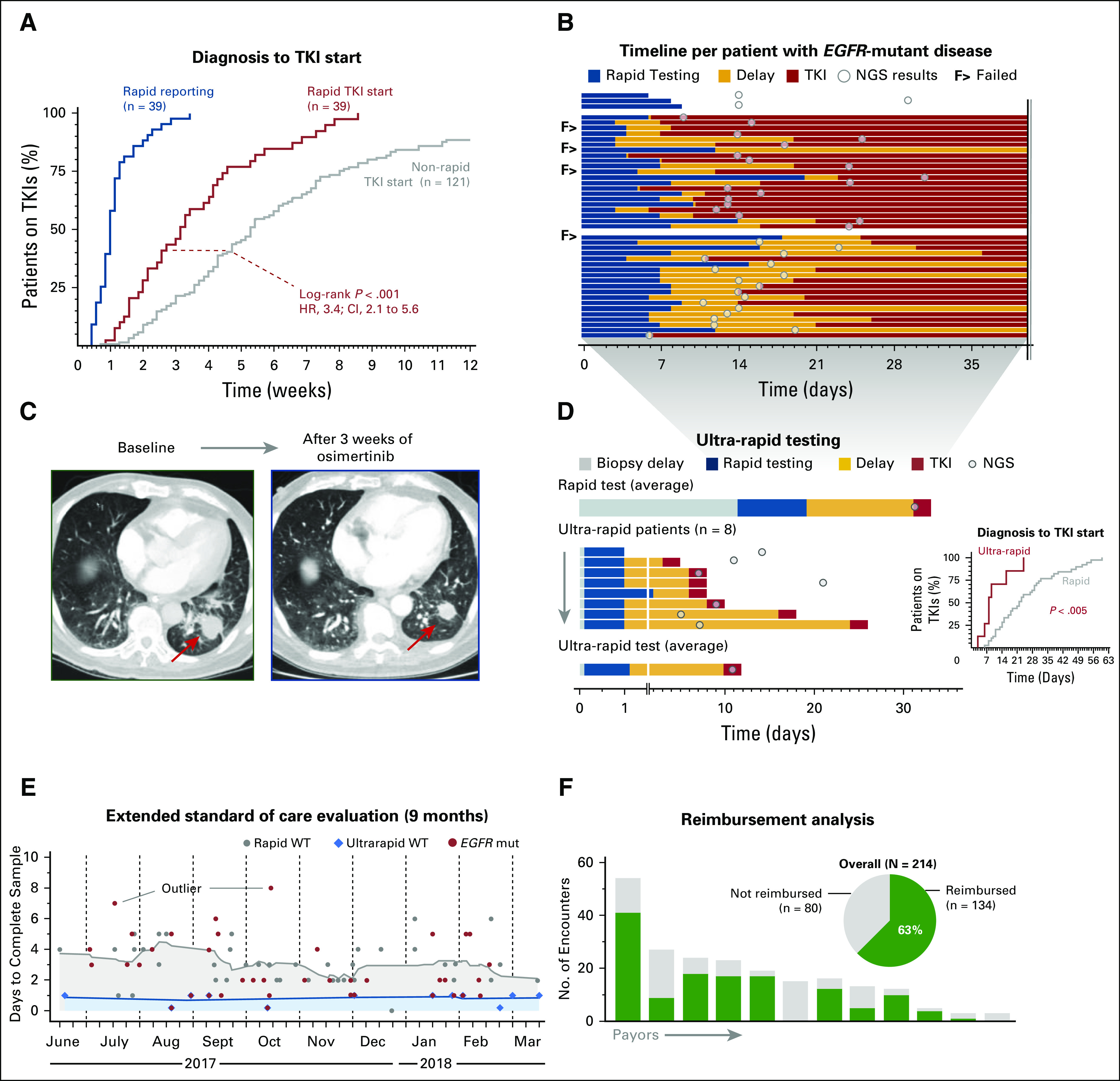
Therapeutic and clinical utility of rapid EGFR genotyping. (A) Event curve that shows rapid EGFR test reporting times and a comparison of the tyrosine kinase inhibitor (TKI) initiation times (relative to date of diagnosis) for patients in the rapid and historical cohorts. (B) Timeline of 43 patients with EGFR-mutant lung cancer. The top three patients did not receive epidermal growth factor receptor (EGFR)–directed therapy during the follow-up period. The second block shows the 49% of patients (n = 17 of 35 patients) with EGFR-mutant disease who started a TKI before next-generation sequencing (NGS) results were available. The third block shows patients who initiated EGFR-directed therapy after NGS results were available. (C) Response to the EGFR inhibitor osimertinib in a patient with non–small-cell lung cancer who underwent ultra-rapid EGFR testing: (left) pretreatment image and (right) response after 3 weeks; arrow indicates primary tumor. (D) Comparison of rapid test times (average) and the eight patients tested with the ultra-rapid protocol (Fig 1C); inset shows event curve comparison of time to initiation of TKI between the rapid and ultra-rapid subsets. (E) Turnaround times for rapid (gray) and ultra-rapid (blue) workflows in a 9-month extended standard-of-care evaluation phase; red, cases with an EGFR mutation. Outliers in reporting times are due to delays in block retrieval or repeated testing. (F) Reimbursement analysis: pie chart depicts the overall frequency of reimbursement; columns illustrate the payor-based number of reimbursed encounters. mut, mutated; WT, wild type.
