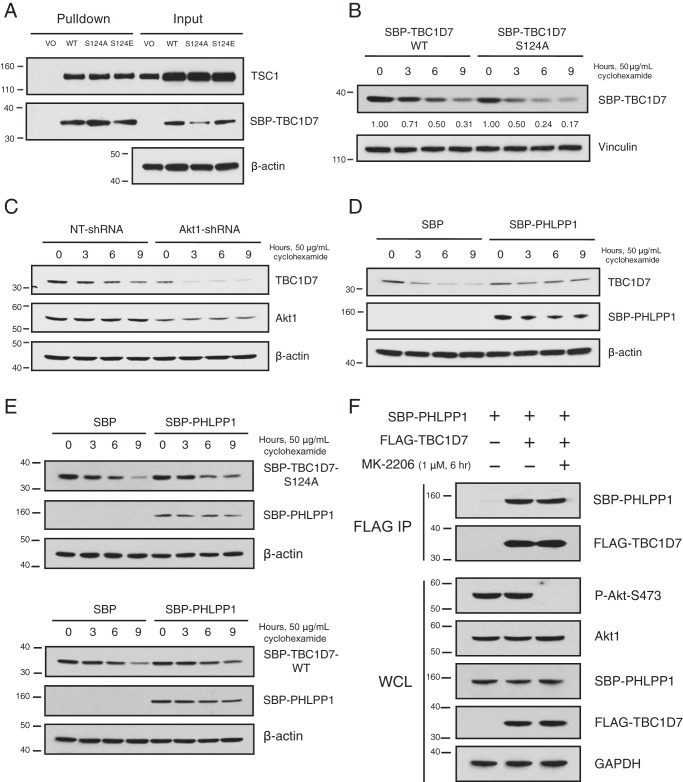
Figure 5.
Ser-124 phosphorylation stabilizes TBC1D7. A, 293T cells were transfected with either SBP vector or SBP-TBC1D7 WT, S124A, S124E expression plasmids. Lysates were subject to pulldown analysis with streptavidin beads. Affinity-purified complexes and input whole-cell lysates were resolved on SDS-PAGE and blotted with TSC1, SBP, and β-actin antibodies. B, 293T cells were transfected with either SBP-TBC1D7-WT or -S124A expression plasmids. The following day, each dish was split into four separate dishes, and the day after, they were treated with 50 μg/ml cyclohexamide for various periods of time. Whole-cell lysates were separated on SDS-PAGE and blotted with SBP and vinculin antibodies. Zero-time points for both WT and S124A SBP-TBC1D7 were set to 1; relative expression of SBP-TBC1D7 proteins is indicated, as determined by densitometric analysis. Values were normalized to vinculin loading control. C, 293T cells stably expressing either NT- or Akt1-shRNA were treated with 50 μg/ml cyclohexamide for various periods of time. Whole-cell lysates were separated on SDS-PAGE and blotted with TBC1D7, Akt1, and β-actin antibodies. D, 293T cells were transfected with either SBP vector or SBP-PHLPP1 expression plasmids. The following day, each dish was split into four separate dishes, and the day after, they were treated with 50 μg/ml cyclohexamide for various periods of time. Whole-cell lysates were separated on SDS-PAGE and blotted with TBC1D7, SBP, and β-actin antibodies. E, 293T cells were co-transfected with SBP vector or SBP-PHLPP1 and SBP-TBC1D7-S124A (top) or SBP-TBC1D7-WT (bottom). The following day, each dish was split into four separate dishes, and the day after, they were treated with 50 μg/ml cyclohexamide for various periods of time. Whole-cell lysates were separated on SDS-PAGE and blotted with SBP and β-actin antibodies. F, 293T cells were co-transfected with either vector or FLAG-TBC1D7 and SBP-PHLPP1 expression plasmids. 42 h after transfection, cells were treated with either DMSO vehicle of MK-2206 (1 μm) for 6 h. Lysates were immunoprecipitated with FLAG antibody–conjugated beads. Immunoprecipitates (IP) and input whole-cell lysates (WCL) were resolved on SDS-PAGE and blotted with SBP, FLAG, P-Akt-Ser-473, and GAPDH antibodies. The P-Akt-Ser-473 blot was stripped and reprobed with an Akt1 antibody.