Figure 8.
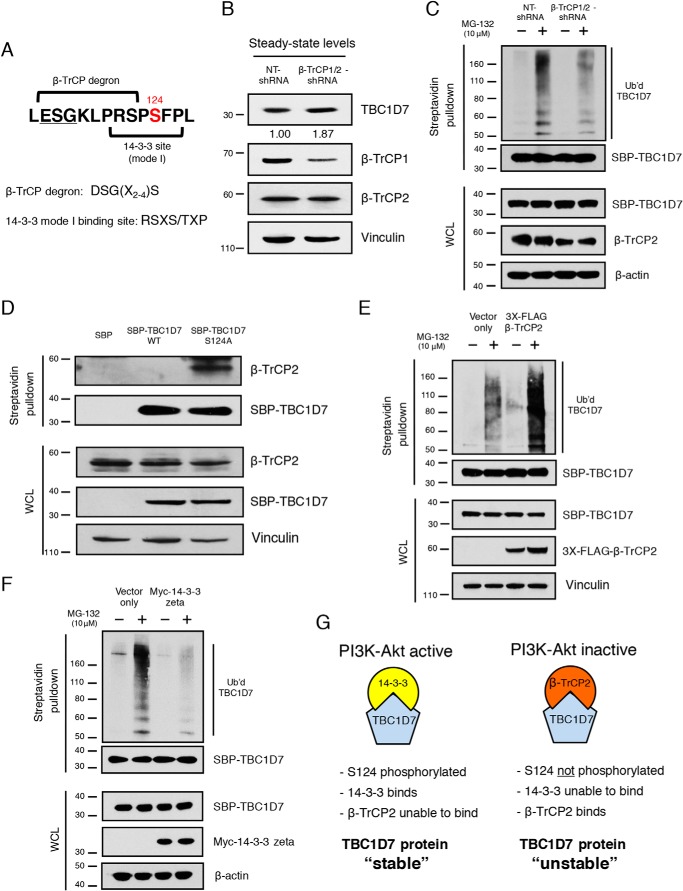
β-TrCP2 regulates TBC1D7 ubiquitination and stability. A, diagram of TBC1D7 primary sequence indicating overlapping β-TrCP degron and 14-3-3 mode I binding site. B, whole-cell lysates from 293T cells stably expressing either nontargeting (NT) or β-TrCP1/2-shRNA were resolved on SDS-PAGE and blotted with TBC1D7, β-TrCP1, β-TrCP2, and vinculin antibodies. Relative expression of endogenous TBC1D7 proteins is indicated, as determined by densitometric analysis. Values were normalized to vinculin loading control. The expression level of endogenous TBC1D7 from nontargeting shRNA-expressing control cells was set to 1. C, 293T cells stably expressing either NT- or β-TrCP1/2-shRNA were co-transfected with HA-ubiquitin and SBP-TBC1D7. Cells were treated or not with 10 μg/ml MG-132 for 4 h. Lysates were subject to pulldown analysis using streptavidin beads. Affinity-purified complexes and input whole-cell lysates (WCL) were resolved on SDS-PAGE and blotted with HA, SBP, β-TrCP2, and β-actin antibodies. D, 293T cells were transfected with either SBP, SBP-TBC1D7-WT, or SBP-TBC1D7-S124A expression plasmids. Lysates were subject to pulldown analysis using streptavidin-agarose beads. Affinity-purified complexes were resolved on SDS-PAGE and blotted with β-TrCP2, SBP, and vinculin antibodies. E, 293T cells were co-transfected with either HA-ubiquitin, SBP-TBC1D7, and empty vector or HA-ubiquitin, SBP-TBC1D7, and 3XFLAG-β-TrCP2 expression plasmids. Cells were treated, or not, with 10 μg/ml MG-132 for 4 h. Lysates were subject to pulldown analysis using streptavidin beads. Affinity-purified complexes and input whole-cell lysates were resolved on SDS-PAGE and blotted with HA, SBP, FLAG, and vinculin antibodies. F, 293T cells were co-transfected with either HA-ubiquitin, SBP-TBC1D7, and empty vector or HA-ubiquitin, SBP-TBC1D7, and Myc-14-3-3ζ expression plasmids. Cells were treated or not with 10 μg/ml MG-132 for 4 h. Lysates were subject to pulldown analysis using streptavidin beads. Affinity-purified complexes and input whole-cell lysates were resolved on SDS-PAGE and blotted with HA, SBP, Myc, and β-actin antibodies. G, diagram representing the role of TBC1D7 Ser-124 as a “phospho-switch” and controlling the stability of TBC1D7 protein. Phosphorylation of Ser-124, via PI3K-Akt signaling, determines binding of 14-3-3 and, thus, binding of β-TrCP2. Ser-124 phosphorylation-dependent binding of TBC1D7 controls the stability of TBC1D7 outside of the TSC complex.