Figure 1.
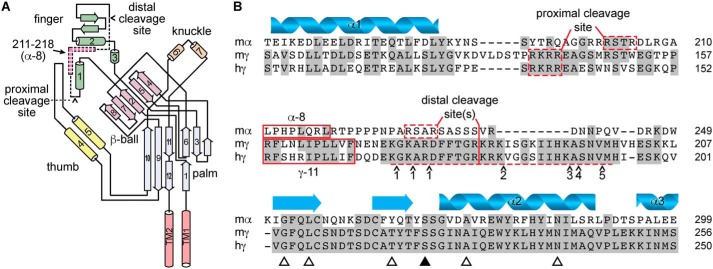
Autoinhibitory tracts in the α and γ subunits are not conserved. A, schematic of ENaC α subunit structure based on ASIC1 structure, adapted from Ref. 40. Cylinders and arrows indicate α-helices and β-strands, respectively, and are numbered to be consistent with the structure of ASIC1 (31). Proximal and distal cleavage sites are indicated. Dashed section of schematic indicates autoinhibitory tract liberated by double cleavage; pink section within indicates key inhibitory LPHPLQRL sequence, corresponding to sequence of α-8. B, sequence alignment of mouse α (mα), mouse γ (mγ), and human γ (hγ) ENaC subunits. Predicted secondary structure of the α subunit is shown. Proximal and distal cleavage sites flank the imbedded inhibitory tracts, which are poorly conserved across subunits. Synthetic peptides corresponding to key sequences in the α and γ subunit inhibitory tracts (i.e. α-8 and γ-11) inhibit channel currents. Several proteases can cleave the γ subunit distal to the imbedded inhibitory tract in the γ subunit, including matriptase/CAP3 at a number of sites (41), and the following proteases at sites indicated by ∧: 1) TMPRSS4/channel activating protease 2 (CAP2) (42), 2) prostasin/channel activating protease 1 (CAP1) (2) and kallikrein (43), 3) plasmin (44), 4) pancreatic elastase, and 5) neutrophil elastase (45). Open triangles indicate sites mutated for experiments in Fig. 4. The closed triangle indicates the site mutated in Fig. 5.