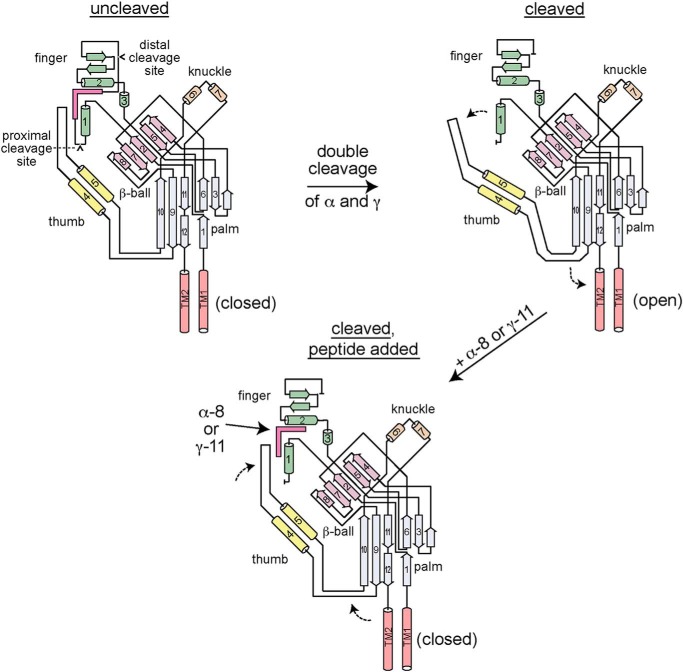
Figure 6.
Model of ENaC proteolytic activation and autoinhibitory tract-derived peptide inhibition. ENaC α and γ subunits are synthesized with autoinhibitory tracts flanked by cleavage sites (upper left). The autoinhibitory tracts facilitate interactions between the finger (β2–α1 loop, helix α2 and preceding β-strands) and thumb domains (α4–α5 loop) that favor a closed conformation of the channel pore. Upon double cleavage of either subunit, the respective autoinhibitory tract is removed, releasing interactions between the top of the thumb domain and the finger domain (upper right). The new arrangement at the finger–thumb domain interface may alter the conformation of the β9–α4 and α5–β10 loops, which interact with the pore forming transmembrane helices (TM2 and TM2). The new structural arrangement favors the open conformation of the channel pore. Addition of either α-8 or γ-11 recapitulates key interactions at the respective finger–thumb domain interface that inhibit the channel, reversing the functional effect of double cleavage.