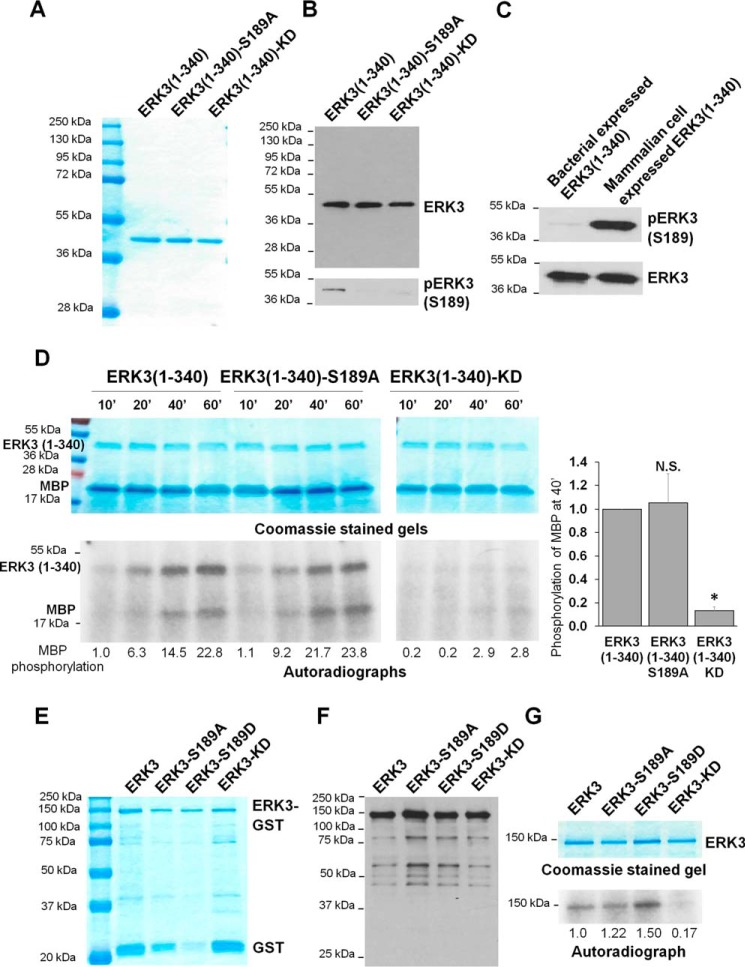
Figure 5.
The S189A mutation does not affect the in vitro kinase activity of recombinant ERK3 protein expressed in bacteria. A, purification of recombinant His-ERK3(1–340) proteins expressed in bacteria. The proteins were purified using Ni-NTA resin and analyzed by SDS-PAGE and subsequent Coomassie Blue staining. The molecular mass of protein markers is indicated. B, His-ERK3(1–340) proteins expressed in bacteria were subjected to Western blot analysis using either an anti-ERK3 mAb that targets an N-terminal epitope in ERK3 (top panel) or an anti-phospho-ERK3 (S189) antibody (bottom panel). C, Western blot analysis of His-ERK3(1–340) protein expressed in bacteria and HA-ERK3(1–340) expressed in 293T cells using anti-ERK3 and anti-phospho-ERK3 (Ser189) antibodies. D, in vitro kinase assay of bacterially expressed ERK3(1–340) proteins. 1 μg of each purified protein was incubated with 2 μg of recombinant MBP and [γ-32P]ATP for different times (minutes). The samples were analyzed by SDS-PAGE, followed by Coomassie staining (above) and autoradiography (below). Quantification of MBP phosphorylation by WT or mutant ERK3(1–340) proteins is shown below the autoradiograph. For the purpose of comparison, the normalized phosphorylation level of MBP by WT ERK3(1–340) at 10 min was arbitrarily set as 1.0. Quantification of MBP phosphorylation after kinase reaction for 40 min (40′) is presented on the right. The MBP phosphorylation level by WT ERK3(1–340) was arbitrarily set as 1.0. The bar graph represents the mean ± S.E. of three independent experiments. *, p < 0.05; N.S., not significantly different by one-way ANOVA. E, purification of recombinant His-ERK3-GST proteins expressed in bacteria. The proteins were purified using GSH beads, followed by elution with reduced GSH. The eluted proteins were analyzed by SDS-PAGE, followed by Coomassie Blue staining. F, Western blot analysis of the purified His-ERK3-GST proteins using an anti-ERK3 antibody that targets an N-terminal epitope in ERK3. G, in vitro kinase assay of bacterially expressed recombinant His-ERK3-GST proteins. 350 ng of purified ERK3 protein (WT or mutant) was incubated with [γ-32P]ATP in kinase assay buffer for measuring autophosphorylation. Protein samples were analyzed by SDS-PAGE and Coomassie staining (top) and autoradiography (bottom). Quantification of ERK3 phosphorylation is shown below the autoradiograph; the normalized phosphorylation level of WT ERK3 was arbitrarily set as 1.0.