The aim of this study was to describe the etiology and outcome of short-term peripheral venous catheter (PVC)-related bloodstream infections (PVCRBSI) in a 25-year period (1992 to 2016) and to identify predictive factors of Gram-negative PVCRBSI. This was a prospective observational study including all episodes of PVCRBSI.
KEYWORDS: Gram negative, bacteremia, bloodstream infections, peripheral venous catheter, phlebitis
ABSTRACT
The aim of this study was to describe the etiology and outcome of short-term peripheral venous catheter (PVC)-related bloodstream infections (PVCRBSI) in a 25-year period (1992 to 2016) and to identify predictive factors of Gram-negative PVCRBSI. This was a prospective observational study including all episodes of PVCRBSI. A multivariate logistic regression model adjusted for calendar year was built to explore factors associated with a Gram-negative bacterial etiology. Over the study period, 711 episodes of PVCRBSI were identified. Incidence rate of PVCRBSI increased from 0.06 to 0.13 episodes/1,000 patient-days. A Gram-negative bacterial etiology was demonstrated in 162 (22.8%) episodes. There was a significant increase in the proportion of Gram-negative infections (22.6% in 1992 to 1996 versus 33.2% in 2012 to 2016). Independent predictive factors of Gram-negative PVCRBSI were the following: being in the hospital for more than 7 days with a catheter in situ for more than 3 days (adjusted odds ratio [aOR], 1.80; 95% confidence interval [CI], 1.20 to 2.69), surgery in the previous month (aOR, 2.39; 95% CI, 1.40 to 4.09), and antimicrobial treatment with beta-lactams (aOR, 1.80; 95% CI, 1.16 to 2.78). In conclusion, we reported an increase in the prevalence of Gram-negative PVCRBSI over the last 25 years. Factors associated with a Gram-negative bacterial etiology were being in the hospital for more than 7 days with a catheter in situ for more than 3 days, having undergone surgery, and having received antimicrobial treatment with beta-lactams.
INTRODUCTION
Short-term peripheral venous catheters (PVCs) are among the most frequently used medical devices, and recent studies estimated the prevalence of hospitalized patients with an indwelling PVC between 23% and 78% (1–5). Two recent literature reviews (6, 7) describe an incidence rate of PVC-related bloodstream infections (PVCRBSI) of 0.5 (95% confidence interval [CI], 0.2 to 0.7) per 1,000 days of PVC use or between 0.02 and 0.2 per 1,000 patient days. Currently, evidence-based internationally endorsed guidelines regarding the management of PVCRBSI are not available, and in the vast majority of cases, the management of these infectious complications is based on the recommendations for central venous catheter (CVC)-related bloodstream infections (CVCRBSI) (8). These guidelines suggest universal empirical antibiotic coverage active against staphylococci, while empirical treatment for Gram-negative bacilli is suggested only in patients with sepsis, neutropenia, a femoral catheter, or a known distal focus of Gram-negative bacillus infection. However, a growing number of reports are highlighting the increase in Gram-negative bacteria as the etiology of CVCRBSI (9–12), a finding which has yet to be fully explored for PVCRBSI.
Given the high number of patients at risk and the associated morbidity and mortality, estimated to be as high as 18% (13), a comprehensive assessment of the characteristics of PVCRBSI seems to be of utmost importance in order to guide the management of this complication.
The aim of our study was to describe the incidence, etiology, clinical characteristics, and outcomes of patients with PVCRBSI admitted over a 25-year period (1992 to 2016) in a large tertiary university hospital and to identify predictive factors of Gram-negative PVCRBSI.
(Part of this research was presented at the 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, 21 to 24 April 2018.)
RESULTS
Patient characteristics, incidence rate, and outcomes.
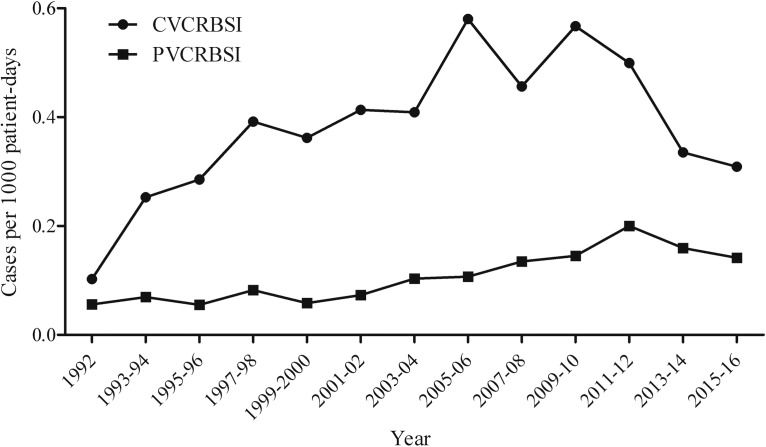
During the study period (1992 to 2016), 27,386 episodes of bacteremia were registered. Among those, 3,842 (14.0%) were classified as catheter-related bloodstream infections, of which 3,337 (12.2%) were of monomicrobial etiology. Of all monomicrobial catheter-related bloodstream infections, 2,626 (9.6%) were CVCRBSI, and 711 (2.6%) were PVCRBSI. Patient characteristics are detailed in Table 1. The overall incidence rate of PVCRBSI increased from 0.06 episodes/1,000 patient days in 1992 to 0.13 episodes/1,000 patient days in 2016; interestingly, in the same period we also detected an increase in the incidence rate of CVCRBSI from 0.10 episodes/1,000 patient days in 1992 to 0.26 episodes/1,000 patient days in 2016 (Fig. 1). The prevalence of PVCRBSI was higher in the first week after hospital admission (54.9% of the total cases), and 28.3% of patients were diagnosed within the first 3 days after catheter placement.
TABLE 1.
Characteristics of patients with PVCRBSI
| Parameter | Value for the parameter (n = 711)a |
|---|---|
| Median age (range [yr]) | 67 (55–77) |
| Male sex | 467 (65.7) |
| Phlebitis | 636 (89.5) |
| Catheter culture | |
| Negative | 12 (1.7) |
| Positive | 154 (21.7) |
| Not performed | 545 (76.7) |
| Prior hospital admission | 99 (13.9) |
| Days since hospital admission | |
| Median no. of days (range) | 7 (4–12) |
| 0–7 | 390 (54.9) |
| 8–14 | 161 (22.6) |
| 15–21 | 60 (8.4) |
| >21 | 68 (9.6) |
| Unknown | 32 (4.5) |
| Days since catheter placement | |
| Median no. of days (range) | 5 (3–7) |
| 0–3 | 201 (28.3) |
| 4–6 | 245 (34.5) |
| 7–9 | 122 (17.2) |
| 10–12 | 46 (6.5) |
| 13–15 | 15 (2.1) |
| 16–18 | 6 (0.8) |
| >19 | 7 (1.0) |
| Unknown | 69 (9.7) |
| Fever | 690 (97.0) |
| Shock | 19 (2.7) |
| Prior surgery | 77 (10.8) |
| Prior nonsurgical invasive procedure | 81 (11.4) |
| Urinary catheter | 133 (18.7) |
| Cirrhosis | 102 (14.3) |
| Diabetes | 163 (22.9) |
| Pulmonary disease | 57 (8.0) |
| HIV infection | 23 (3.2) |
| Chronic kidney disease | 67 (9.4) |
| Cardiac disease | 249 (35.0) |
| Hematological malignancy | 53 (7.5) |
| Solid organ malignancy | 112 (15.8) |
| Neutropenia | 14 (2.0) |
| Solid organ transplant | 32 (4.5) |
| Corticosteroid therapy | 129 (18.1) |
| McCabe, ultimately or rapidly fatal outcome | 278 (39.1) |
| 7-Day mortality | 26 (3.7) |
| 30-Day mortality | 53 (7.5) |
| Persistent bacteremia | 33 (4.6) |
| Correct empirical treatment | 386 (54.3) |
| Prior antibiotic treatment | 169 (23.8) |
| Microbial etiology | |
| Gram-positive cocci | 533 (75.0) |
| Staphylococcus aureus | 327 (46.0) |
| Coagulase-negative staphylococci | 178 (25.0) |
| Enterococcus spp. | 26 (3.7) |
| Gram-negative bacilli | 162 (22.8) |
| Klebsiella spp. | 40 (5.6) |
| Pseudomonas aeruginosa | 32 (4.5) |
| Escherichia coli | 29 (4.1) |
| Enterobacter spp. | 28 (3.9) |
| Serratia spp. | 5 (0.7) |
| Acinetobacter spp. | 5 (0.7) |
| Candida spp. | 9 (1.3) |
Values are given as number of patients (percentage) unless otherwise indicated.
FIG 1.
Incidence rate of catheter-related bloodstream infections.
Overall, 10.5% of patients did not present with phlebitis and were diagnosed as a result of a positive catheter tip culture in the absence of other apparent infectious foci. Phlebitis was less common in patients with PVCRBSI due to Gram-negative bacilli than in patients with a Gram-positive bacterial etiology (80.9% versus 92.4%; P value of <0.001); interestingly, while presentation with phlebitis was not significantly different among patients infected with Gram-negative bacteria, patients with Staphylococcus aureus PVCRBSI had a higher proportion of phlebitis than patients infected with other Gram-positive cocci (94.8% versus 88.7%; P value of 0.009). Nevertheless, a significant difference in rates of phlebitis occurrence between infections with Gram-negative bacteria and those with Gram-positive bacteria was still evident even after excluding Staphylococcus aureus from the pool of Gram-positive bacteria (80.9% versus 88.7%; P value of 0.033).
PVC tip culture was more frequently performed in patients with a Gram-negative PVCRBSI (32.1% versus 20.6%; P value of <0.001) than in those with Gram-positive PVCRBSI. Specifically, PVC tip culture was less frequently performed in patients with Staphylococcus aureus bacteremia (19.0% versus 26.9%; P value of 0.001), possibly because in these cases the diagnosis was more evident for the physician in charge. Characteristics of patients with a positive PVC tip culture and patients without one are presented in Table 2. Phlebitis, a requirement for PVCRBSI diagnosis, was present in all patients without a PVC tip culture and in only 51.3% of patients with a positive PVC tip culture. Patients with a positive PVC tip culture were more likely to have a longer hospital stay and have a PVC in situ for a longer period of time. Cirrhosis, diabetes, and solid-organ malignancies were less frequent in patients with a positive PVC tip culture, while cardiac disease was more common than in patients without a PVC tip culture. The proportion of patients with Gram-negative PVCRBSI was higher in subjects with a positive PVC tip culture (31.2% versus 20.2%; P value of 0.004).
TABLE 2.
Comparison of patients with PVCRBSI with a positive PVC tip culture and patients with PVCRBSI without an available PVC tip culture
| Parameter | Value for the groupa |
P valueb | |
|---|---|---|---|
| Positive PVC tip culture (n = 154) | PVC tip culture not performed (n = 545) | ||
| Median age (range [yr]) | 67 (50–77) | 67 (55–78) | 0.346 |
| Male sex | 96 (62.3) | 362 (66.4) | 0.290 |
| Phlebitis | 79 (51.3) | 545 (100.0) | <0.001 |
| Prior hospital admission | 21 (13.6) | 77 (14.1) | 0.877 |
| Days since hospital admission | |||
| Median no. of days (range) | 8 (5–13) | 6 (4–11) | 0.002 |
| 0–7 | 64 (41.6) | 317 (58.2) | <0.001 |
| >7 or unknown | 90 (58.4) | 228 (41.8) | |
| Days since catheter placement | |||
| Median no. of days (range) | 5 (4–8) | 5 (3–7) | 0.014 |
| 0–3 | 28 (18.2) | 169 (31.0) | 0.002 |
| >3 or unknown | 126 (81.8) | 376 (69.0) | |
| Fever | 149 (96.8) | 529 (97.1) | 0.842 |
| Shock | 4 (2.6) | 13 (2.4) | 0.880 |
| Prior surgery | 22 (14.3) | 53 (9.7) | 0.106 |
| Prior nonsurgical invasive procedure | 14 (9.1) | 65 (11.9) | 0.326 |
| Urinary catheter | 29 (18.8) | 100 (18.3) | 0.892 |
| Cirrhosis | 7 (4.5) | 92 (16.9) | <0.001 |
| Diabetes | 25 (16.2) | 134 (24.6) | 0.029 |
| Pulmonary disease | 13 (8.4) | 44 (8.1) | 0.883 |
| HIV infection | 8 (5.2) | 15 (2.8) | 0.134 |
| Chronic kidney disease | 8 (5.2) | 56 (10.3) | 0.054 |
| Cardiac disease | 75 (48.7) | 169 (31.0) | <0.001 |
| Hematological malignancy | 10 (6.5) | 43 (7.9) | 0.563 |
| Solid organ malignancy | 11 (7.1) | 101 (18.5) | 0.001 |
| Neutropenia | 4 (2.6) | 10 (1.8) | 0.551 |
| Solid organ transplant | 4 (2.6) | 27 (5.0) | 0.210 |
| Corticosteroid therapy | 28 (18.2) | 100 (18.3) | 0.962 |
| McCabe, ultimately or rapidly fatal outcome | 44 (28.6) | 229 (42.0) | 0.003 |
| Previous ABTc | 38 (24.7) | 128 (23.5) | 0.759 |
| Beta-lactams | 26 (16.9) | 80 (14.7) | 0.501 |
| Penicillins | 13 (8.4) | 39 (7.2) | 0.591 |
| Third-generation cephalosporins | 11 (7.1) | 32 (5.9) | 0.562 |
| Carbapenems | 1 (0.6) | 17 (3.1) | 0.088 |
| Aminoglycosides | 4 (2.6) | 2 (0.4) | 0.023 |
| Glycopeptides | 3 (1.9) | 10 (1.8) | 0.927 |
| Fluoroquinolones | 9 (5.8) | 44 (8.1) | 0.356 |
| Linezolid | 2 (1.3) | 6 (1.1) | 0.839 |
| Current ABT | 23 (14.9) | 113 (20.7) | 0.108 |
| Betalactams | 15 (9.7) | 61 (11.2) | 0.609 |
| Penicillins | 7 (4.5) | 30 (5.5) | 0.639 |
| Third-generation cephalosporins | 7 (4.5) | 21 (3.9) | 0.699 |
| Carbapenems | 0 (0.0) | 5 (0.9) | 0.233 |
| Aminoglycosides | 2 (1.3) | 7 (1.3) | 0.989 |
| Glycopeptides | 1 (0.6) | 5 (0.9) | 0.999 |
| Fluoroquinolones | 5 (3.2) | 40 (7.3) | 0.068 |
| Linezolid | 0 (0.0) | 1 (0.2) | 0.999 |
Values are given as number of patients (percentage) unless otherwise indicated.
Significant values are in boldface.
ABT, antibiotic therapy.
Correct empirical treatment was administered in 54.3% of patients, and 4.6% of the patients had persistent bacteremia under active antibiotic treatment. Incorrect empirical antibiotic treatment was more frequently administered in patients with PVCRBSI due to Pseudomonas aeruginosa (37.5% versus 55.1%, P value = 0.051) and Enterobacteriaceae resistant to third-generation cephalosporins (27.8% versus 55.0%; P value of 0.022) and methicillin-resistant coagulase-negative staphylococci (24.2% versus 58.9%; P value of <0.001).
Overall in-hospital mortality rates at 7 and 30 days after the diagnosis of PVCBRSI were 3.7% and 7.5%, respectively. No difference in 7- and 30-day overall in-hospital mortality rates was observed between patients with Gram-negative versus Gram-positive PVCRBSI, while persistent bacteremia was more frequent among patients with Gram-positive infections (Table 3). Interestingly, overall in-hospital mortality was not significantly different from that in patients with CVCRBSI (4.6% and 8.7% at 7 and 30 days; P values of 0.273 and 0.296, respectively).
TABLE 3.
Characteristics of patients with PVCRBSI due to Gram-positive and Gram-negative bacteria
| Parameter | Value for the groupa |
P valueb | |
|---|---|---|---|
| Gram-positive infection (n = 540) | Gram-negative infection (n = 162) | ||
| Median age (range [yr]) | 67 (55–77) | 66 (52–78) | 0.480 |
| Male sex | 348 (64.4) | 111 (68.5) | 0.339 |
| Phlebitis | 499 (92.4) | 131 (80.9) | <0.001 |
| PVC culture | 0.009 | ||
| Negative | 8 (1.5) | 4 (2.5) | |
| Positive | 103 (19.1) | 48 (29.6) | |
| Not performed | 429 (79.4) | 110 (67.9) | |
| Prior hospital admission | 74 (13.7) | 24 (14.8) | 0.720 |
| Days since hospital admission | |||
| Median no. of days (range) | 6 (4–10) | 8 (5–17) | <0.001 |
| 0–7 | 319 (59.1) | 69 (42.6) | <0.001 |
| >7 or unknown | 221 (40.9) | 93 (57.4) | |
| Days since catheter placement | |||
| Median no. of days (range) | 5 (3–7) | 5 (3–7) | 0.129 |
| 0–3 | 162 (30.0) | 38 (23.5) | 0.106 |
| >3 or unknown | 378 (70.0) | 124 (76.5) | |
| Fever | 527 (97.6) | 154 (95.1) | 0.114 |
| Shock | 13 (2.4) | 6 (3.7) | 0.407 |
| Prior surgery | 40 (7.4) | 36 (22.2) | <0.001 |
| Prior non-surgical invasive procedure | 58 (10.7) | 23 (14.2) | 0.227 |
| Urinary catheter | 93 (17.2) | 38 (23.5) | 0.074 |
| Cirrhosis | 92 (17.0) | 9 (5.6) | <0.001 |
| Diabetes | 130 (24.1) | 31 (19.1) | 0.190 |
| Pulmonary disease | 44 (8.1) | 13 (8.0) | 0.960 |
| HIV infection | 18 (3.3) | 4 (2.5) | 0.580 |
| Chronic kidney disease | 58 (10.7) | 9 (5.6) | 0.049 |
| Cardiac disease | 200 (37.0) | 47 (29.0) | 0.061 |
| Hematological malignancy | 44 (8.1) | 9 (5.6) | 0.273 |
| Solid organ malignancy | 82 (15.2) | 28 (17.3) | 0.519 |
| Neutropenia | 10 (1.9) | 3 (1.9) | 0.999 |
| Solid organ transplant | 27 (5.0) | 5 (3.1) | 0.306 |
| Corticosteroid therapy | 92 (17.0) | 33 (20.4) | 0.331 |
| McCabe, ultimately or rapidly fatal outcome | 227 (42.0) | 45 (27.8) | 0.001 |
| 7-Day mortality | 21 (3.9) | 5 (3.1) | 0.635 |
| 30-Day mortality | 40 (7.4) | 12 (7.4) | 0.999 |
| Persistent bacteremia | 30 (5.6) | 2 (1.2) | 0.021 |
| Correct empirical treatment | 305 (56.5) | 80 (49.4) | 0.111 |
| Previous ABTc | 107 (19.8) | 55 (34.0) | <0.001 |
| Beta-lactams | 61 (11.3) | 43 (26.5) | <0.001 |
| Penicillins | 22 (4.1) | 26 (16.0) | <0.001 |
| Third-generation cephalosporins | 29 (5.4) | 14 (8.6) | 0.128 |
| Carbapenems | 6 (1.1) | 12 (7.4) | <0.001 |
| Aminoglycosides | 6 (1.1) | 1 (0.6) | 0.999 |
| Glycopeptides | 8 (1.5) | 5 (3.1) | 0.190 |
| Fluoroquinolones | 36 (6.7) | 14 (8.6) | 0.391 |
| Linezolid | 3 (0.6) | 5 (3.1) | 0.019 |
| Current ABT | 94 (17.4) | 40 (24.7) | 0.039 |
| Beta-lactams | 51 (9.4) | 22 (13.6) | 0.130 |
| Penicillins | 20 (3.7) | 15 (9.3) | 0.004 |
| Third-generation cephalosporins | 22 (4.1) | 4 (2.5) | 0.343 |
| Carbapenems | 3 (0.6) | 2 (1.2) | 0.327 |
| Aminoglycosides | 7 (1.3) | 1 (0.6) | 0.689 |
| Glycopeptides | 6 (1.1) | 1 (0.6) | 0.999 |
| Fluoroquinolones | 34 (6.3) | 11 (6.8) | 0.822 |
| Linezolid | 0 (0.0) | 1 (0.6) | 0.231 |
Values are given as number of patients (percentage) unless otherwise indicated.
Significant values are in boldface.
ABT, antibiotic therapy.
Microbiological etiology.
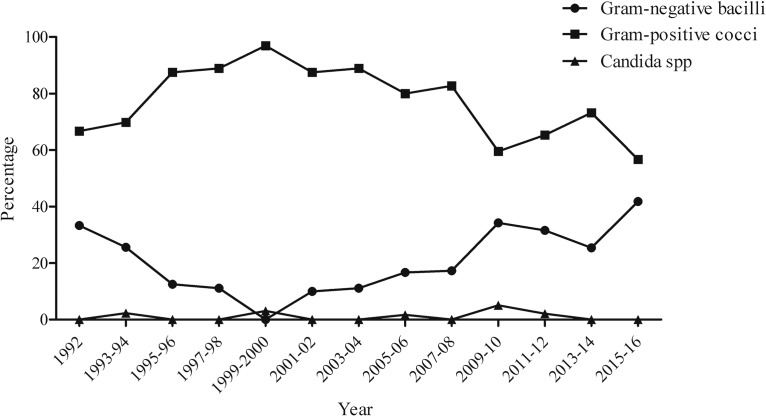
During the study period, 533 cases (75.0%) were caused by Gram-positive cocci, 162 (22.8%) were caused by Gram-negative bacilli, and 9 (1.3%) were caused by Candida spp. Microbiological etiology of PVCRBSI is described in Table 1. Notably, over the study period (Fig. 2 and 3) there was a significant increase in the proportion of PVCRBSI due to Gram-negative bacilli (P value of <0.001), with a corresponding reduction of Gram-positive coccus etiology (P value of <0.001), while the proportion of PVCRBSI due to Candida spp. remained stable over time (P value of 0.865). The majority of Gram-negative PVCRBSI were due to Klebsiella spp. (24.7%), followed by Pseudomonas aeruginosa (19.8%) and Escherichia coli (17.9%). The proportion of isolates resistant to third-generation cephalosporins was 17.5% among Klebsiella spp., 17.2% for Escherichia coli, and 21.4% among Enterobacter spp., while 9.4% and 15.6% of Pseudomonas aeruginosa isolates were resistant to ceftazidime and carbapenems, respectively. Among Gram-positive cocci, 61.4% of episodes were due to Staphylococcus aureus (9.5% resistant to methicillin), 33.4% were due to coagulase-negative staphylococci (53.4% resistant to methicillin), and 4.9% were due to Enterococcus spp. (26.9% resistant to ampicillin).
FIG 2.
Incidence rate of PVCRBSI according to microbial etiology.
FIG 3.
Microbial etiology of PVCRBSI.
Predictive factors for developing Gram-negative PVCRBSI.
Demographic, epidemiological, and clinical characteristics of patients with PVCRBSI due to Gram-negative and Gram-positive bacteria are compared in Table 3. Notably, a Gram-negative bacterial etiology was more frequent among patients who were admitted to the hospital for more than 7 days and who had undergone surgical intervention in the previous 30 days. Moreover, PVCBRSI due to Gram-negative bacteria was more common in patients who had received antimicrobial treatment in the previous month, especially with beta-lactams and linezolid, and in those receiving antibiotics at the time of PVCRBSI diagnosis. On the other hand, a Gram-positive bacterial etiology was more frequent in patients with phlebitis, cirrhosis, and chronic kidney failure and in those with a worse McCabe prognostic score.
After multivariate logistic regression analysis adjusted for calendar year (Table 4), independent predictive factors of developing Gram-negative PVCRBSI were the following: being in the hospital for more than 7 days with a catheter in situ for more than 3 days (adjusted odds ratio [aOR], 1.80; 95% CI, 1.20 to 2.69), having undergone surgery in the previous month (aOR, 2.39; 95% CI, 1.40 to 4.09), and having received antimicrobial treatment with beta-lactams in the previous month or concomitantly with the episode of bacteremia (aOR, 1.80; 95% CI, 1.16 to 2.78). Factors associated with a lower risk of Gram-negative PVCRBSI were presenting with phlebitis (aOR, 0.35; 95% CI, 0.20 to 0.63), having cirrhosis (aOR, 0.40; 95% CI, 0.19 to 0.86), or a cardiovascular comorbidity (aOR, 0.56; 95% CI, 0.36 to 0.85) and a worse McCabe prognostic score (aOR, 0.57; 95% CI, 0.37 to 0.88). The Hosmer-Lemeshow goodness-of-fit test yielded a nonsignificant result (P value of 0.303). No collinearity was detected in the multivariable model (variance inflation factor [VIF] range, 1.020 to 2.163).
TABLE 4.
Logistic regression analysis for factors associated with Gram-negative bacillus bacteremia in patients with PVCRBSI
| Factor | OR (95% CI) | P value for unadjusted ORa | aOR (95% CI) | P value for aORa |
|---|---|---|---|---|
| Year of diagnosis | <0.001 | <0.001 | ||
| 1992–1996 | Reference | Reference | ||
| 1997–2001 | 0.226 (0.087–0.590) | 0.002 | 0.277 (0.101–0.763) | 0.013 |
| 2002–2006 | 0.566 (0.285–1.126) | 0.105 | 0.883 (0.416–1.876) | 0.747 |
| 2007–2011 | 1.268 (0.712–2.260) | 0.420 | 1.821 (0.951–3.486) | 0.070 |
| 2012–2016 | 1.701 (0.958–3.018) | 0.070 | 2.322 (1.199–4.495) | 0.012 |
| Hospital stay of >7 days with a catheter in situ for >3 days | 1.951 (1.367–2.785) | <0.001 | 1.799 (1.204–2.688) | 0.004 |
| Prior surgery | 3.540 (2.172–5.769) | <0.001 | 2.388 (1.396–4.085) | <0.001 |
| Prior or current beta-lactam therapy | 2.401 (1.626–3.547) | <0.001 | 1.797 (1.161–2.782) | 0.009 |
| Phlebitis | 0.368 (0.224–0.606) | <0.001 | 0.351 (0.197–0.625) | <0.001 |
| Cirrhosis | 0.288 (0.142–0.586) | 0.001 | 0.399 (0.186–0.855) | 0.018 |
| Cardiac disease | 0.702 (0.480–1.028) | 0.069 | 0.556 (0.364–0.851) | 0.007 |
| McCabe, ultimately or rapidly fatal outcome | 0.522 (0.356–0.765) | 0.001 | 0.569 (0.368–0.878) | 0.011 |
Values in boldface are significant.
Finally, a subgroup analysis was performed in patients with a positive PVC culture. The only two factors associated with a Gram-negative bacterial etiology were the presence of phlebitis (aOR, 0.383; 95% CI, 0.186 to 0.787) and previous surgery (aOR, 2.480; 95% CI, 0.946 to 6.500), even though the latter did not reach statistical significance, probably due to the smaller sample size.
DISCUSSION
In this prospective observational study, we described the characteristics and outcomes of 711 peripheral venous catheter-related bloodstream infections diagnosed over 25 years at our center. To our knowledge, this is the largest prospective study analyzing PVCRBSI. The incidence rate of PVCRBSI doubled over time, reaching 0.13 episodes/1,000 patient days in 2016, half the incidence rate of CVCRBSI. However, a trend toward a progressive reduction has been observed during recent years, which could be attributable to the implementation since 2012 of a program similar to Bacteremia Zero for CVC (14, 15) that was implemented in our intensive care unit (ICU) in 2010 and significantly reduced the incidence of CVCRBSI (Fig. 1). Importantly, even though patients with a CVC were more severely ill and had a higher number of comorbidities, overall in-hospital mortality rates at both 7 and 30 days did not differ significantly between groups. This finding prompts a high level of suspicion for the diagnosis of PVCRBSI, which must be managed rapidly and appropriately.
Over the last 25 years, we observed a significant increase in PVCRBSI due to Gram-negative bacteria. This change in the epidemiology has been described in several reports regarding CVCRBSI (9–12, 16, 17), but data concerning PVCRBSI are scarce. A recent study by Sato et al. described a similar prevalence of Gram-negative infections (35.8%) in the 2010-2015 period (18) while a Spanish nationwide study conducted only in internal medicine departments showed a Gram-negative bacillus prevalence of 11.1% (19).
In our study, factors associated with a Gram-negative bacterial etiology were prior surgical intervention, antibiotic treatment in the previous 30 days or during the episode of bacteremia, and having a PVC in situ for more than 3 days in patients being admitted to the hospital for more than 7 days.
Prior surgical intervention has already been described as a risk factor for Gram-negative bacteremia (20). We may speculate that in this subset of patients, a higher frequency of Gram-negative bacteremia (even without overt infection) may be a predisposing factor of catheter colonization and subsequent infection. All the patients included in the study were evaluated for surgery-related infectious complications, and patients who had such a complication were excluded even if they had concomitant phlebitis due to the presence of another source of infection.
To our knowledge no studies have evaluated the relationship between length of hospital stay with a catheter in situ and the risk of Gram-negative infection. This finding may be related to a change in the Gram-negative proportion of bacteria in the skin microbiota of the hospitalized patients (21), but further studies are needed in order to better elucidate the role of skin flora in the pathophysiology of catheter-related infection (22).
Calendar year was also found to be an independent predictor of Gram-negative bacteremia, suggesting that some epidemiological or environmental factors not included in our analysis may be related to the increase of Gram-negative PVCRBSI seen in recent years; nevertheless, the implementation of prevention bundles which may be more effective in reducing Gram-positive infections (23, 24) may partially explain this finding.
Clinical signs of phlebitis were present in almost 90% of patients with a confirmed PVCRBSI; however, 1 out of 10 patients did not present with local inflammation, and the diagnosis was made only thanks to a positive catheter tip culture yielding the same pathogen isolated in the peripheral blood cultures. In the clinical setting, this result reflects the importance of having a dedicated team to search for the source of all episodes of bacteremia. Current clinical practice guidelines (8) do not specifically refer to PVC tip culture and suggest systematic removal and culture of the catheter in case of suspected intravascular catheter-related bloodstream infection. Moreover, data regarding the proportion of PVC tip cultures performed in patients with suspected PVCRBSI are scarce, and when reported the proportion is frequently under 50% (18, 19). Given these findings, we may conclude that a systematic removal and culture of PVC should be performed not only in all patients with clinical apparent phlebitis but also in subjects with systemic signs of infection and without a known septic focus. Interestingly, the proportion of cases without phlebitis was higher in patients with Gram-negative PVCRBSI, as one-fifth of the patients did not have signs of local inflammation. Considering all this information, we may also infer that the actual incidence of Gram-negative PVCRBSI may be underestimated.
This study has several limitations. First, as a study from a single institution, the generalizability of our results is difficult; however, several reports from other centers also highlighted an increase in the incidence of Gram-negative infections. Second, the incidence rate in our study was estimated using the number of patient days as a reference unit as data regarding catheter days were not available. Indeed, knowing the actual number of days at risk would have been optimal to characterize the incidence of PVCRBSI in our population. Third, internationally validated criteria for the diagnosis of PVCRBSI are not currently available, and thus there is a risk of over- or underdiagnosis. Fourth, PVC tip culture was performed only in a minority of patients, possibly resulting in overdiagnosis of PVCRBSI (for example, in cases of chemical phlebitis). Nevertheless, we think that having an infectious diseases team dedicated to follow and review each patient ensured proper diagnosis and management. Finally, as already stated, given the relatively high number of patients who were diagnosed with PVCRBSI thanks to a positive tip culture in the absence of phlebitis (especially in the case of Gram-negative bacterial etiology), it is possible that the actual incidence rate of PVCRBSI may be higher than what we reported as cases of bacteremia of unknown focus without signs of phlebitis at the PVC insertion site might not have been considered possible PVCRBSI.
In conclusion, in our study we reported an increase in the incidence rate and prevalence of Gram-negative PVCRBSI. Remarkably, approximately one out of five patients with Gram-negative PVCRBSI did not show signs of phlebitis. PVC tip culture should be performed in all patients with suspected PVCRBSI and in patients with bacteremia without a clinically apparent focus, even in the absence of phlebitis. As PVCRBSI are common and potentially fatal, physicians need to maintain a high degree of suspicion in order to promptly diagnose and treat patients with this infectious complication.
MATERIALS AND METHODS
Study design.
This study was a prospective observational study including all episodes of short-term peripheral venous catheter-related monomicrobial bacteremia diagnosed from January 1992 to December 2016 at the Hospital Clínic in Barcelona. Our center is a 700-bed tertiary university hospital covering an urban population of 550,000 inhabitants. The decision regarding the necessity and the type of short-term peripheral venous catheter was made by the patient's physician.
In our center, a dedicated team of infectious diseases physicians and microbiologists identifies and prospectively follows up all patients with bacteremia until death or 30 days after diagnosis, whichever occurs first. For each patient, a set of selected demographic, clinical, biochemical, microbiological, and antimicrobial treatment variables are collected and entered into a specific database.
Standard procedures of catheter insertion and management in our center were previously described (25). Peripheral catheter site and adequate functioning are inspected in each nursing shift or when otherwise needed, as in the case of apparent malfunctioning or due to patient complaints. Consequently, peripheral catheters are removed in the case of signs of complications or if no longer needed. Predefined scheduled changes of peripheral catheter are never included as a routine practice since the literature is controversial regarding the potential benefits of this procedure (26). PVCRBSI incidence rate is defined as the number of episodes/1,000 patient days.
STROBE (27) recommendations were followed in order to strengthen the reporting of the study.
Definition of study variables.
Since a consensus regarding the definition of PVCRBSI is lacking, we applied the currently accepted definition of catheter-related bloodstream infections (8). Thus, PVCRBSI was defined as the presence of one positive peripheral blood culture and a positive semiquantitative or quantitative catheter tip culture that grew the same microorganism found in the peripheral blood culture; in the absence of a positive catheter tip culture, patients were diagnosed with PVCRBSI when there were local signs of phlebitis and absence of other evident infectious foci. Specifically, in the case of bacteremia due to coagulase-negative staphylococci without an available catheter tip culture, at least two sets of positive blood cultures drawn from different venipuncture localizations were required to make a diagnosis of PVCRBSI. All the cases of PVCRBSI were reviewed and discussed by the dedicated team of infectious diseases physicians and microbiologists responsible for treating and following the patient throughout the hospital admission.
Phlebitis was defined as the presence of two or more of the following signs or symptoms on examination of the catheter insertion site: pain (either reported by the patient or tenderness elicited by palpation), erythema, swelling, purulence, and a palpable venous cord, as previously described (25). The following data at the time of bloodstream infection diagnosis were obtained for all patients and prospectively recorded in the registry: age, sex, admission date, date of catheter insertion and number of days of catheter in situ, preexisting comorbidities, McCabe score (28), and presence of an indwelling urinary catheter. Moreover, data regarding administration of immunosuppressive therapy (20 mg of daily prednisone or equivalent), surgery, or other nonsurgical invasive procedures and antibiotic use within the month preceding the bacteremia were collected. Active empirical antibiotic therapy was defined as a treatment started within the first 24 h of the index blood cultures, having in vitro activity against the bacteria isolated. The presence of the following clinical factors when the blood cultures were drawn was also documented: local signs of phlebitis, presentation with septic shock (defined according to previous guidelines [29]), and presentation with fever. Persistent bacteremia was defined as the presence of positive blood cultures after initiation of active antibiotic therapy.
Microbiological methods.
Between 1992 and 1997, blood samples were processed by a Bactec NR-730 system (Becton-Dickinson Microbiology System) and incubated routinely for 7 days. The Bactec 9240 system (Becton-Dickinson Microbiology System) was adopted in 1998 and uses an incubation period of 5 days. PVC tip culture was performed using a roll-plate method with a semiquantitative culture as described by Maki et al. (30), followed by catheter tip culture in an enriched thioglycolate medium (BBL), with an incubation time of 72 h at 35°C.
Isolates were identified according to standard techniques, including the matrix-assisted laser desorption–ionization time of flight (MALDI-TOF) technique (since 2012). Antibiotic susceptibility was determined according to the recommendations of the CLSI or EUCAST guidelines available at the time of diagnosis. For statistical analysis, intermediate susceptibility was considered resistance.
Statistical analysis.
Baseline characteristics were compared using a chi-square test or Fisher's exact test for categorical variables and Wilcoxon's rank sum test for continuous variables. A chi-square trend test was used to evaluate differences observed during the study period. A univariate logistic regression analysis was carried out to explore factors associated with Gram-negative etiology of PVCRBSI. A multivariate model was subsequently built by subjecting variables with a univariate P value of <0.20 to a backward stepwise logistic regression procedure. The calibration of the model was assessed by means of the Hosmer-Lemeshow goodness-of-fit test. The presence of collinearity was tested using the variance inflation factor (VIF) and tolerance. Interactions between variables were explored. A subgroup analysis was performed in patients with a positive PVC culture. Continuous variables are reported as median (interquartile range [IQR]); categorical variables are reported as frequency (percentage); logistic regression analysis results are reported as adjusted odds ratios (aOR) with 95% confidence intervals (95% CI). A two-tailed P value of <0.05 was considered significant. Statistical analysis was performed using SPSS, version 20.0 (IBM SPSS, Chicago, IL, USA).
Ethics.
The study was approved by the local ethics committee (HCB/2017/1029). No informed consent was sought since this was an observational study and clinical care was not influenced.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We thank all the patients and all the colleagues of the Infectious Diseases and Microbiology Service of the Hospital Clínic.
REFERENCES
- 1.Humphreys H, Newcombe RG, Enstone J, Smyth ET, McIlvenny G, Fitzpatrick F, Fry C, Spencer RC, Hospital Infection Society Steering Group . 2008. Four country healthcare associated infection prevalence survey 2006: risk factor analysis. J Hosp Infect 69:249–257. doi: 10.1016/j.jhin.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 2.Vandenbos F, Basar A, Tempesta S, Fournier JP, Bertrand F, Vanesland L, Oualid H, Dunais B, Dellamonica P, Roger PM. 2003. Relevance and complications of intravenous infusion at the emergency unit at Nice University Hospital. J Infect 46:173–176. doi: 10.1053/jinf.2002.1101. [DOI] [PubMed] [Google Scholar]
- 3.Zingg W, Pittet D. 2009. Peripheral venous catheters: an under-evaluated problem. Int J Antimicrob Agents 34(Suppl 4):S38–S42. doi: 10.1016/S0924-8579(09)70565-5. [DOI] [PubMed] [Google Scholar]
- 4.Trinh TT, Chan PA, Edwards O, Hollenbeck B, Huang B, Burdick N, Jefferson JA, Mermel LA. 2011. Peripheral venous catheter-related Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 32:579–583. doi: 10.1086/660099. [DOI] [PubMed] [Google Scholar]
- 5.Guembe M, Pérez-Granda MJ, Capdevila JA, Barberán J, Pinilla B, Martín-Rabadán P, Bouza E, NUVE Study Group . 2015. Nationwide study on the use of intravascular catheters in internal medicine departments. J Hosp Infect 90:135–141. doi: 10.1016/j.jhin.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Mermel LA. 2017. Short-term peripheral venous catheter-related bloodstream infections: a systematic review. Clin Infect Dis 65:1757–1762. doi: 10.1093/cid/cix562. [DOI] [PubMed] [Google Scholar]
- 7.Maki DG, Kluger DM, Crnich CJ. 2006. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 81:1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 8.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcos M, Soriano A, Inurrieta A, Martínez JA, Romero A, Cobos N, Hernández C, Almela M, Marco F, Mensa J. 2011. Changing epidemiology of central venous catheter-related bloodstream infections: increasing prevalence of Gram-negative pathogens. J Antimicrob Chemother 66:2119–2125. doi: 10.1093/jac/dkr231. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Créixems M, Muñoz P, Martín-Rabadán P, Cercenado E, Guembe M, Bouza E. 2013. Evolution and aetiological shift of catheter-related bloodstream infection in a whole institution: the microbiology department may act as a watchtower. Clin Microbiol Infect 19:845–851. doi: 10.1111/1469-0691.12050. [DOI] [PubMed] [Google Scholar]
- 11.Braun E, Hussein K, Geffen Y, Rabino G, Bar-Lavie Y, Paul M. 2014. Predominance of Gram-negative bacilli among patients with catheter-related bloodstream infections. Clin Microbiol Infect 20:O627–O629. doi: 10.1111/1469-0691.12565. [DOI] [PubMed] [Google Scholar]
- 12.Gonçalves P, Menezes FG, Toniolo AR, Silva CV, Cardoso MF, Kawagoe JY, Santos CM, Castagna HM, Martino MD, Correa L. 2015. Secular trends in central line-associated bloodstream infection: microbiological pattern of pathogens after preventive measures. Infect Control Hosp Epidemiol 36:1106–1107. doi: 10.1017/ice.2015.128. [DOI] [PubMed] [Google Scholar]
- 13.Pujol M, Hornero A, Saballs M, Argerich MJ, Verdaguer R, Cisnal M, Peña C, Ariza J, Gudiol F. 2007. Clinical epidemiology and outcomes of peripheral venous catheter-related bloodstream infections at a university-affiliated hospital. J Hosp Infect 67:22–29. doi: 10.1016/j.jhin.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel Cl. 2006. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 15.Palomar M, Álvarez-Lerma F, Riera A, Díaz MT, Torres F, Agra Y, Larizgoitia I, Goeschel CA, Pronovost PJ, Bacteremia Zero Working Group . 2013. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU. Crit Care Med 41:2364–2372. doi: 10.1097/CCM.0b013e3182923622. [DOI] [PubMed] [Google Scholar]
- 16.Nemoto T, Kunishima H, Shimizu G, Hirose M, Yamasaki Y, Nishisako H, Takagi T, Matsuda T. 2015. Factors predicting the cause and prognosis of central line-associated bloodstream infections. J Infect Chemother 21:118–122. doi: 10.1016/j.jiac.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Bouza E, Eworo A, Fernández Cruz A, Reigadas E, Rodríguez-Créixems M, Muñoz P. 2013. Catheter-related bloodstream infections caused by Gram-negative bacteria. J Hosp Infect 85:316–320. doi: 10.1016/j.jhin.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Sato A, Nakamura I, Fujita H, Tsukimori A, Kobayashi T, Fukushima S, Fujii T, Matsumoto T. 2017. Peripheral venous catheter-related bloodstream infection is associated with severe complications and potential death: a retrospective observational study. BMC Infect Dis 17:434. doi: 10.1186/s12879-017-2536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guembe M, Pérez-Granda MJ, Capdevila JA, Barberán J, Pinilla B, Martín-Rabadán P, Bouza E. NUVE Study Group 2017. Nationwide study on peripheral-venous-catheter-associated-bloodstream infections in internal medicine departments. J Hosp Infect 97:260–266. doi: 10.1016/j.jhin.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Marschall J, Zhang L, Foxman B, Warren DK, Henderson JP. 2012. Both host and pathogen factors predispose to Escherichia coli urinary-source bacteremia in hospitalized patients. Clin Infect Dis 54:1692–1698. doi: 10.1093/cid/cis252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassir N, Papazian L, Fournier P-E, Raoult D, La Scola B. 2015. Insights into bacterial colonization of intensive care patients' skin: the effect of chlorhexidine daily bathing. Eur J Clin Microbiol Infect Dis 34:999–1004. doi: 10.1007/s10096-015-2316-y. [DOI] [PubMed] [Google Scholar]
- 22.Mermel LA. 2011. What is the predominant source of intravascular catheter infections? Clin Infect Dis 52:211–212. doi: 10.1093/cid/ciq108. [DOI] [PubMed] [Google Scholar]
- 23.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network ( NHSN) Team and participating NHSN facilities. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Holtom P, Vigen C. 2011. Reduction of catheter-related bloodstream infections through the use of a central venous line bundle: epidemiologic and economic consequences. Am J Infect Control 39:640–646. doi: 10.1016/j.ajic.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Martínez JA, Piazuelo M, Almela M, Blecua P, Gallardo R, Rodríguez S, Escalante Z, Robau M, Trilla A. 2009. Evaluation of add-on devices for the prevention of phlebitis and other complications associated with the use of peripheral catheters in hospitalised adults: a randomised controlled study. J Hosp Infect 73:135–142. doi: 10.1016/j.jhin.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 26.Webster J, Osborne S, Rickard C, New K.. 2015. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev 8:CD007798. [DOI] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative 2007. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCabe WR. 1962. Gram-negative bacteremia. Arch Intern Med 110:847–855. doi: 10.1001/archinte.1962.03620240029006. [DOI] [Google Scholar]
- 29.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevranksy JE, Spring CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale J, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup . 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maki DG, Weise CE, Sarafin HW. 1977. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med 296:1305–1309. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]