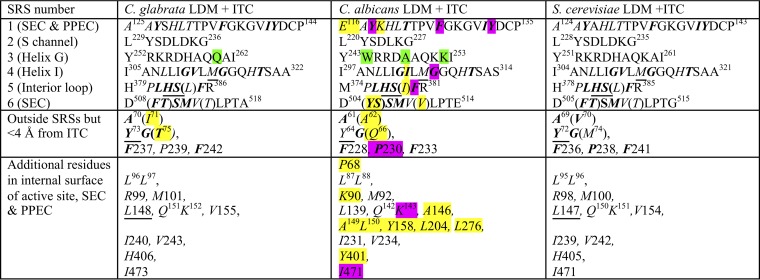
TABLE 3.
Amino acid residues contributing to the LBPa
SRS1 to SRS6 are substrate recognition sites as defined by Warrilow et al. (20). Residues in italics contribute to the interior surface of the LBP, i.e., the active site, the SEC (tunnel 2f [21]), and the PPEC (8). Residues not contributing to the LBP are in normal text. Residues within 4 Å of ITC are in boldface. Residues differing either chemically or in conformation compared with the reference structurally aligned S. cerevisiae residues are highlighted in yellow if within the LBP and in green if not. Nonidentical structurally aligned residues contributing to the LBP within 4 Å of ITC are shown in brackets. Y64 in CaLDM is within 4.1 Å of ITC. We find 48 residues contribute the interior surface of the LBP of CgLDM and ScLDM. CaLDM K118 at the external edge of the PPEC and P68 beside the water-containing pocket in the SEC may also contribute small areas to the LBP, while a further 5 residues may artifactually extend the LBP. Among the residues contributing to the LBP, single mutations at 8 residues (highlighted in purple) have been demonstrated to contribute to azole resistance in C. albicans. No such mutations have been reported for CgLDM. The 6 residues underlined are found in major ascomycete and basidiomycete fungal pathogens but not in their human or plant hosts and have yet to be shown to confer resistance to azole drugs. LBP, ligand binding pocket. SEC, substrate entry channel. PPEC, putative product exit channel.