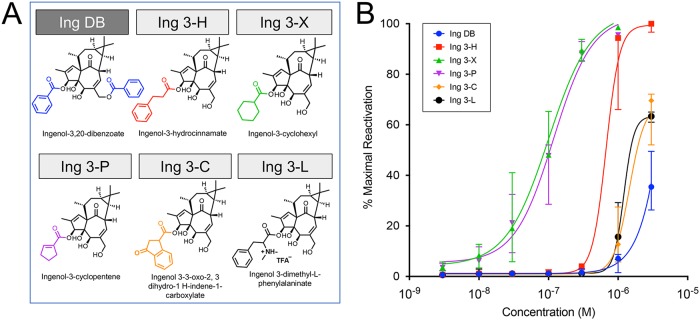
FIG 3.
Latency reversal activity of aromatic and carbocyclic ingenol-C-3-esters. Aromatic and carbocyclic esters were added to the 3-carbon position of the ingenol core molecule (structures, full and abbreviated nomenclature shown in panel A), with the exception of ingenol-3,20-dibenzoate, which was commercially available. Aromatic and carbocyclic ingenol-3-esters were tested for latency reversal efficacy using a latent HIV-1 cell line (J-Lat 10.6) containing a full-length integrated HIV-1 provirus that expresses GFP upon proviral transcription. Compounds were tested across a dose range from 1 nM to 3,000 nM. After 24 h of exposure, flow cytometry was performed to quantify GFP-positive cells, which was compared to positive control (PMA stimulation) to obtain percent maximal HIV-1 reactivation. (B) Fitted dose-response curves with median and 95% confidence intervals are shown based on five independent in vitro experiments.