FIG 4.
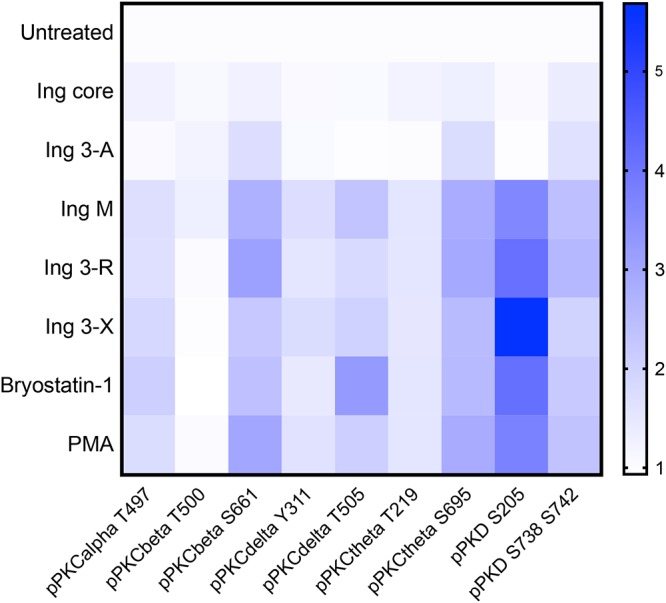
PKC isoform activation after ingenol exposure. Resting primary CD4 cells isolated from HIV-1-uninfected participants (n = 5) underwent a 30-min exposure to ingenol derivatives (100 nM), bryostatin-1 (100 nM), or PMA (10 ng/ml). Cells underwent intracellular staining with anti-pPKC antibodies and were analyzed by flow cytometry. Changes in PKC isoform phosphorylation were quantified by the fold change in mean fluorescence intensity compared to untreated (negative-control) cell cultures (increasing mean fluorescence intensity [MFI] fold change represented above as blue color scale). Ingenol core and Ing 3-A, which have little to no latency reversal activity, did not induce any PKC isoform phosphorylation in resting primary CD4 cells isolated from HIV-1-uninfected participants (n = 5). Highly active ingenol derivatives Ing M, Ing 3-R, and Ing 3-X significantly induced phosphorylation of PKC isoforms PKCβ, PKCδ, PKCθ, and PKD. This PKC isoform phosphorylation pattern did not significantly differ among these ingenols or structurally distinct PKC agonists PMA or bryostatin-1.
