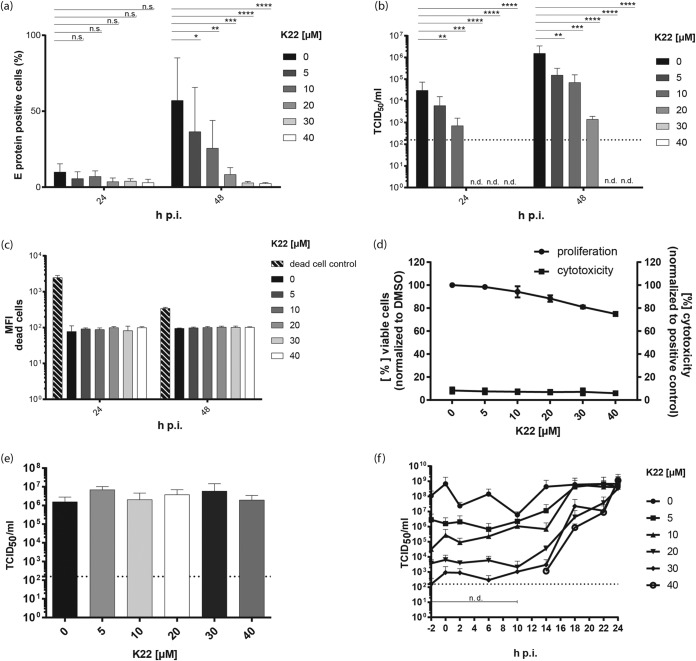
FIG 1.
K22 inhibits ZIKV replication and acts at a postentry stage. Vero cells were treated for 4 h with different concentrations of K22. Cells were infected with ZIKV (MOI = 0.1 TCID50/cell) for 1 h before the medium was removed and medium containing the different concentrations of K22 was added. (a) At 24 h and 48 h p.i., cells were harvested and E protein expression was analyzed by flow cytometry. (b) Viral infectivity was determined by harvesting the supernatant of cells and performing a TCID50 assay on Vero cells. (c) Cells treated with the compound were stained with a live/dead cell marker, and the MFI was determined by flow cytometric analyses. Shaded bars represent the positive control of dead cells. (d) Commercially available cell toxicity assays (cytotoxicity; CytoTox 96 nonradioactive cytotoxicity assay; Promega) as well as viability assays (proliferation; MultiTox-Fluor multiplex cytotoxicity assay; Promega) were performed to determine the cytotoxic effect of K22 on Vero B4 cells at 24 h posttreatment. (e) To evaluate the direct effect of K22 on viral stability, ZIKV in DMEM was incubated for 2 h at room temperature with the different K22 concentrations, and the viral titer was determined as the number of TCID50 per milliliter. (f) Time-of-addition assays were performed to analyze the effect of K22 on the early virus-cell interaction. K22 at various concentrations was added at specific time points relative to the time of infection with ZIKV, and the cell culture supernatants were collected at 24 h p.i. for measurement of virus release. The results represent the mean (bar) + SD (n = 2 or 3). Dotted lines indicate the limit of detection. n.d., not detected. P values were determined by 2-way ANOVA, followed by Dunnett's multiple-comparison test. ****, P ≤ 0.0001; ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05; n.s., not significant.