Figure 3.
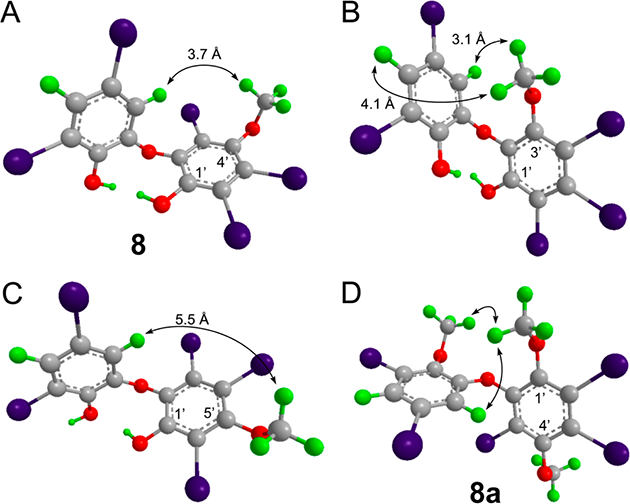
Interproton distances of three possible isomers of 8 and NOEs observed for 8a. Coordinates for the three possible structures of compound 8 were energy minimized using Chem3D, and interproton distances between H-4 and H-6 of ring A and the −OCH3 group in ring B were measured in each and compared to the experimental NOE data (A–C). The isomer shown in B (1′-hydroxy-3′-methyoxy) predicts NOEs that are not observed, and the isomer in C (1′-hydroxy-5′-methyoxy) shows a distance greater than 5.5 Å for an NOE that is present. Panel D shows the NOEs observed for methylated 8a, in agreement with experimental data. 1D NOE spectra are shown in Figure S13.
