Figure 2.
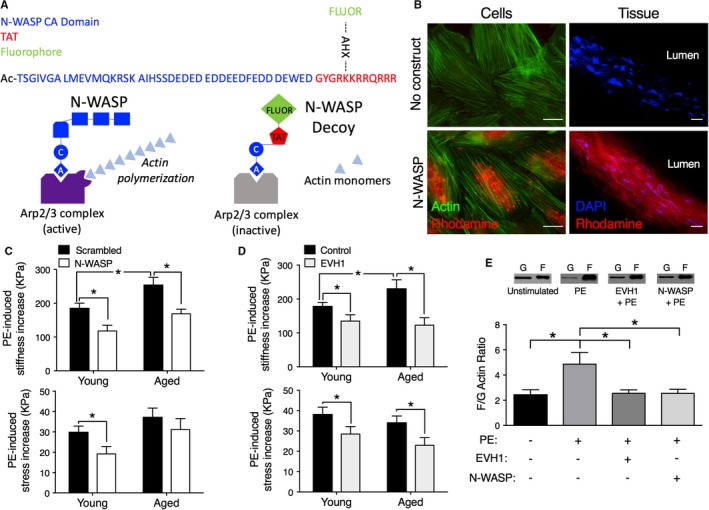
Decoy constructs targeted to actin polymerization molecules decrease aortic stiffness. A, Top, Design of prototype for cell‐permeant decoy peptides. Blue type, protein‐specific domain sequence, acetylated on the N‐terminus to maintain stability. N‐WASP CA dominant negative peptide sequence shown. Red type, sequence derived from the TAT known to be a cell permeation sequence38, 39 added to C‐terminus. Green type, a fluorescent dye (FITC or rhodamine) added to an ε‐aminohexanoic acid (AXH) bridge as a side chain off a lysine of the TAT sequence in order to confirm intracellular loading. The AHX bridge allows the fluorophore to be added without adverse effects on peptide stability. Bottom, Diagram illustrating the method of inhibition by the decoy peptide. The CA domain inhibits the binding of N‐WASP to the Arp2/3 complex and prevents its activation. B, Decoy constructs permeate into aortic cells (left) and tissue (right). Left panels, No added construct (top) or rhodamine‐labeled (red) N‐WASP CA peptide‐loaded cells (bottom). Costained with phalloidin (green) for actin. Incorporated peptide is especially noticeable in the perinuclear region, the thickest part of the cell. Right panels, No added construct (top) or rhodamine‐labeled N‐WASP CA peptide‐loaded into ex vivo aortic tissue (bottom). Tissue costained with DAPI to locate nuclei. Images are obtained at identical microscope and software settings (look‐up tables, offsets, and gains). Scale bars: 20 μm. C, Top panel, Aortic tissues from aged mice show significantly increased active, PE‐induced stiffness compared with those from young mice (black bars). The introduction of the N‐WASP CA domain peptide (white vs black bars) significantly decreases active stiffness (top panel) in both young (3‐month‐old, n=6) and old (24‐29 months, n=6) mice. The peptide decreases PE‐induced stress significantly only in young mice (bottom panel). Asterisks indicate significant differences (P<0.05) from scrambled peptides. D, Top panel, Aortic tissues from aged mice show significantly increased active, PE‐induced stiffness compared with those from young mice (black bars). Decoy construct targeted to EVH1 decreases PE‐mediated aortic stiffness (top panel) and stress (bottom panel) (tan vs black bars) ex vivo in young (n=12) and aged (n=7) mice. Asterisks indicate significant differences P<0.05 from control peptides. E, Inhibition of PE‐induced actin polymerization (n=7, young adult, 3‐ to 4‐month‐old mice) by the N‐WASP CA domain peptide (n=8) and EVH1 construct (n=8), compared with baseline values (n=7), demonstrate on‐target effects. PE indicates phenylephrine.
