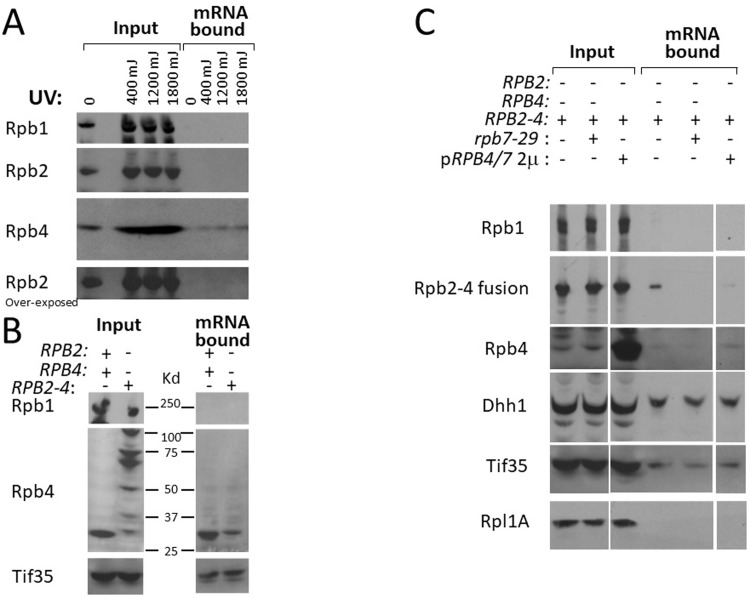
Fig 5. Endogenous Rpb4, Rpb2-Rpb4 fusion protein and the fusion-derived Rpb4 bind poly(A)+ RNAs.
(A) Rpb4, but not Rpb2, binds poly(A)+ RNA. Live WT cells were irradiated with increasing doses of UV radiation, as indicated. RNPs were extracted from equal amount of cells and the poly(A)+ RNA was purified [27]. Proteins, which were captured by equal amount of mRNA (see Materials and methods), were analyzed by Western blotting. The membrane was cut at ~ 100 Kd into two pieces; the high MW portion was reacted with anti-Rpb2, whereas the low MW portion reacted with anti-Rpb4 antibodies. The high MW portion was then reacted with anti-Rpb1 Abs (anti-CTD). A 5-fold overexposure of the Rpb2 signal is shown in the lower panel. (B) Rpb4 derived from cleavage of the Rpb2-Rpb4 fusion protein binds poly(A)+ RNA. WT and RPB2-RPB4 cells were irradiated with 1200 mJ of UV. Poly(A)+ RNA was purified under denaturing conditions and RNA-associated proteins were analyzed as in (A). The membrane was cut as in A and reacted with the either anti-Rpb1 or anti-Rpb4 Abs. The membrane piece with the low MW proteins was later reacted with anti-Tif35 Abs. Tif35 is an eIF3 component and is shown as a loading control. (C) The Rpb2-Rpb4 fusion protein binds poly(A)+ RNAs in an Rpb4/7-dependent manner. The RPB2-RPB4 strain was transformed with a high-copy plasmid encoding an Rpb7 mutant defective in Rpb4-binding (Rpb7-29) or a high-copy plasmid carrying both RPB4 and RPB7 ORFs including their respective 5’ and 3’ non-coding regions (pRPB4/7). Poly(A)+ RNA was purified and RNA-associated proteins were analyzed as in (A).