Figure 1.
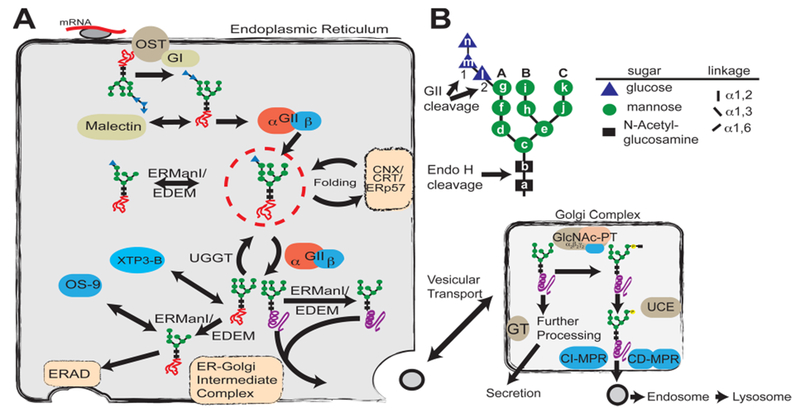
Glycan processing in the secretory pathway. (A) Role of glycosylation in the determination of protein fate in the ER and Golgi complex. A preassembled Glc3Man9GlcNAc2 (G3M9) is transferred by oligosaccharyltransferase (OST) from a dolichol-PP-glycan donor to a consensus sequence asparagine-X-serine/threonine (X can be any amino acid except proline) in a nascent polypeptide chain (red line). Glucosidase I (GI) removes the first glucose residue (residue n), producing a G2M9 glycan that is a substrate for malectin as well as glucosidase II (GII). GII catalyzes the hydrolysis of the second glucose (residue m), leaving G1M9 on the newly synthesized glycoprotein. The protein is then recognized by calnexin/calreticulin (CNX/CRT), which in turn recruits ERp57, a disulfide protein isomerase. GII cleaves the innermost glucose (residue l), making it a target for UDP-Glc:glycoprotein glucosyltransferase (UGGT), which may add residue l depending on the folding status of the glycoprotein. Throughout the folding process, proteins are also targets for ER mannosidases. Folded proteins (purple helix) shuttle to the ER–Golgi intermediate complex for subsequent vesicular transport, whereas non-native structures (orange scrambled lines) are reglucosylated by UGGT for recognition by the CNX/CRT cycle or further processed by mannosidases for removal by the endoplasmic reticulum-associated degradation (ERAD) pathway. (B) Structure of the glycan transferred to proteins during N-glycosylation. Individual hexose moieties are labeled a–n in the order of synthesis of the dolichol-PP-glycan. First and second cleavages by GII, the endo-β-N-acetylglucosaminidase H (Endo H) cleavage site, and arms A–C of the glycan are indicated.
