Figure 2.
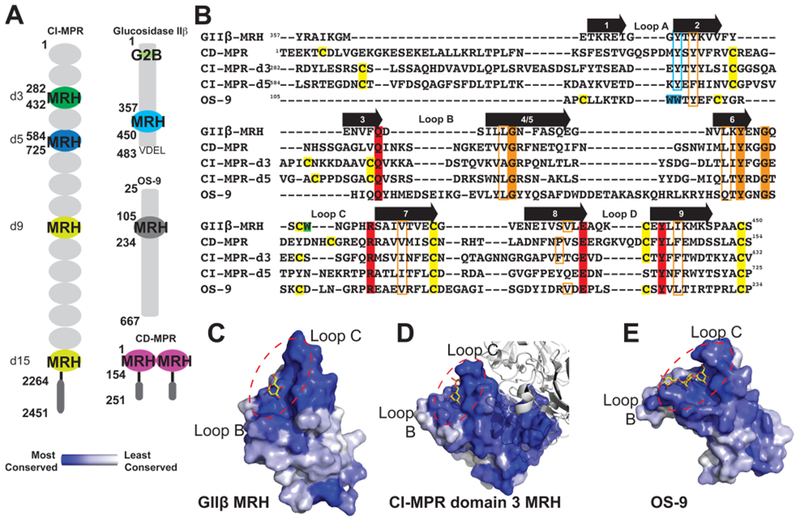
Comparison of glycan binding MRH domains from GIIβ, CD-MPR, CI-MPR, and OS-9. (A) Schematic representation comparing the domain organization of the ER resident protein GIIβ subunit from Schizosaccharomyces pombe and human OS-9 with the bovine CD-MPR and CI-MPR that recycle between the Golgi complex, endosomes, and cell surface. The transmembrane and cytosolic region of the CD-MPR and CI-MPR are indicated by a horizontal black line and dark gray oval, respectively. The mature proteins, lacking the signal sequence, are depicted. The numbering of residues begins at the mature protein for GIIβ, CD-MPR, and CI-MPR and at the initiator methionine for OS-9. The carbohydrate binding MRH domains of CI-MPR are highlighted (domain 3, d3; domain 5, d5; domain 9, d9; domain 15, d15). The structures are known for the MRH domains of domains 3 and 5 of CI-MPR, CD-MPR, OS-9, and GIIβ, and their amino acid sequences are shown in panel B. (B) Structure-based sequence alignment of MRH domains from MRH-bearing proteins. Residues essential for Man-6-P binding (Q, R, E, and Y) in MPRs are highlighted in red, and tryptophan residues of OS-9 involved in Manα1,6Man linkage recognition are highlighted in cyan. The corresponding tyrosine residue in GIIβ-MRH, domain 3 and 5 of the CI-MPR, and the CD-MPR are boxed in cyan. W409 of GIIβ is highlighted in green. The newly identified conserved glycine and tyrosine residues are highlighted in orange, while conserved hydrophobic residues are boxed in orange. Cysteine residues are highlighted in yellow. The molecular surface of the MRH domain of GIIβ (C), domain 3 of CI-MPR (D), and OS-9 (E) are shown colored by species conservation as determined by ConSurf (http://consurf.tau.ac.il/).50 The ligand binding region in panels C–E is circled with a dashed red line.
