Figure 5.
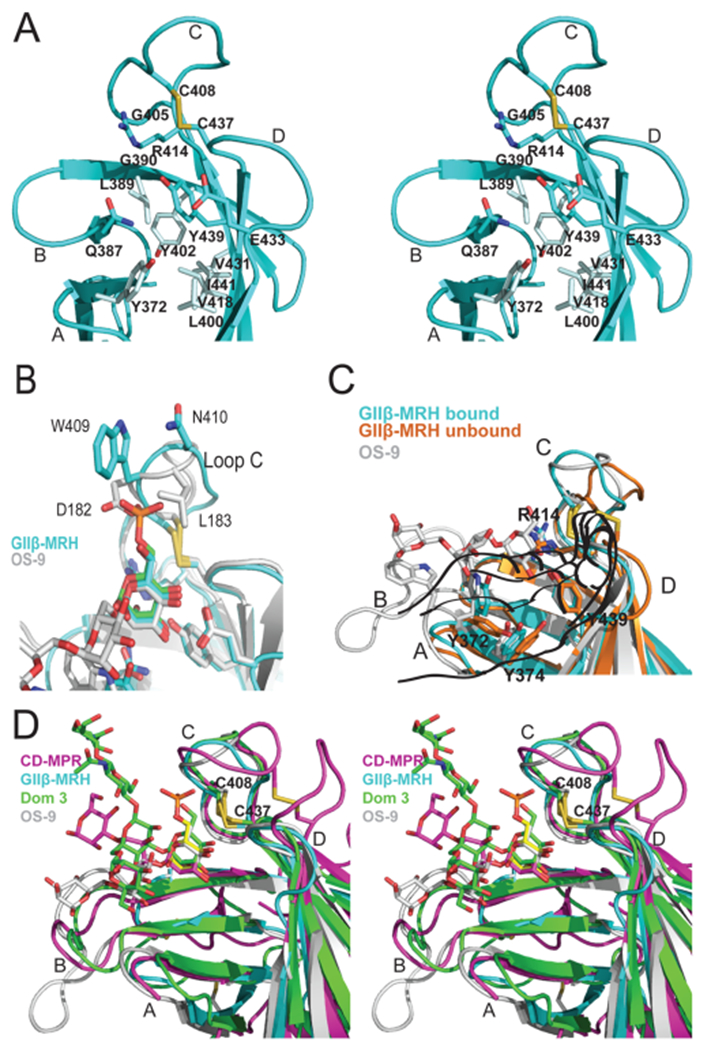
Specificity of the binding sites of MRH domains. (A) Ribbon diagram depicting a close-up stereoview of the binding pocket of the GIIβ MRH domain. Loops A–D along with the hydrophobic residues discussed in the text are labeled. The cysteine residues involved in a disulfide bridge (C408–C437, yellow) and side chains (sticks) of residues in the binding pocket are also shown. (B) Ribbon diagram of the loop C region of the MRH domains of GIIβ (cyan) and OS-9 (gray) with their respective ligands colored according to protein and the superimposed Man-6-P ligand colored green. Disulfide bridges are colored yellow. (C) Ribbon diagram of the GIIβ MRH domain in the absence (orange) and presence (cyan) of mannose. For comparison, the positioning of OS-9’s ligand (trisaccharide in the crystal structure) and the corresponding loop with W118 are colored gray. Disulfide bridges are colored yellow. (D) Ribbon diagram depicting a close-up stereoview of the binding pocket showing the superimposition of CD-MPR bound to pentamannosyl phosphate (magenta), domain 3 of CI-MPR (Dom3) bound to the asparagine-linked oligosaccharide of a crystallographic neighbor (green), OS-9 bound to a mannopentose (gray), and GIIβ (cyan) bound to mannose (yellow) MRH domains. Loops A–D are labeled, and the disulfide bridge is shown (C408–C437, yellow).
