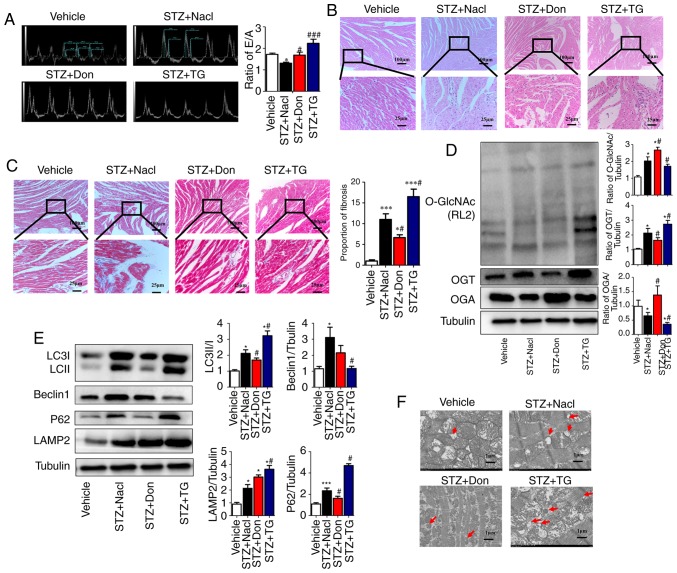
Figure 2.
Increased O-GlcNAc modification in vivo inhibits autophagic flux and aggravates myocardial function in type I DM rats. Rats were randomly divided into five groups (n≥6 in each group) for treatment: Vehicle, STZ, STZ+NaCl, STZ+Don, and STZ+TG. (A) M-mode echocardiography showed the left ventricular diastolic function as the ratio of E/A. Morphological changes in the myocardium were assessed by (B) hematoxylin and eosin staining and (C) Masson staining (scale bar=100 and 25 µm); (D) Expression levels of O-GlcNAc (RL2), OGA and OGT in each group were detected by western blot analysis. (E) Western blot analysis was used to detect the expression of autophagy markers LC3II/I, Beclin1, P62, and LAMP2. Data are expressed as the mean ± standard error of the mean (n≥6). *P<0.05 and ***P<0.001, vs. Vehicle group; #P<0.05 and ###P<0.001, vs. STZ+NaCl group. Tubulin was the loading control. (F) Transmission electron microscopy showed the autophagosomes, which are characterized by a double-layer membrane structure, as indicated by red arrows (scale bar=1 µm). O-GlcNAc, O-linked β-N-acetylglucosamine; STZ, streptozotocin; Don, 6-diazo-5-oxo-L-norleucine; TG, thiamet G; OGT, O-GlcNAc transferase; OGA, O-GlcNAcase; LAMP2, lysosome-associated membrane protein 2; LC3, microtubule-associated protein 1 light chain 3α; E/A, left ventricular filling peak velocity/atrial contraction flow peak velocity.